Abstract
BACKGROUND
Endovascular therapy is increasingly used after the administration of intravenous tissue plasminogen activator (t-PA) for patients with moderate-to-severe acute ischemic stroke, but whether a combined approach is more effective than intravenous t-PA alone is uncertain.
METHODS
We randomly assigned eligible patients who had received intravenous t-PA within 3 hours after symptom onset to receive additional endovascular therapy or intravenous t-PA alone, in a 2:1 ratio. The primary outcome measure was a modified Rankin scale score of 2 or less (indicating functional independence) at 90 days (scores range from 0 to 6, with higher scores indicating greater disability).
RESULTS
The study was stopped early because of futility after 656 participants had undergone randomization (434 patients to endovascular therapy and 222 to intravenous t-PA alone). The proportion of participants with a modified Rankin score of 2 or less at 90 days did not differ significantly according to treatment (40.8% with endovascular therapy and 38.7% with intravenous t-PA; absolute adjusted difference, 1.5 percentage points; 95% confidence interval [CI], −6.1 to 9.1, with adjustment for the National Institutes of Health Stroke Scale [NIHSS] score [8–19, indicating moderately severe stroke, or ≥20, indicating severe stroke]), nor were there significant differences for the predefined subgroups of patients with an NIHSS score of 20 or higher (6.8 percentage points; 95% CI, −4.4 to 18.1) and those with a score of 19 or lower (−1.0 percentage point; 95% CI, −10.8 to 8.8). Findings in the endovascular-therapy and intravenous t-PA groups were similar for mortality at 90 days (19.1% and 21.6%, respectively; P = 0.52) and the proportion of patients with symptomatic intracerebral hemorrhage within 30 hours after initiation of t-PA (6.2% and 5.9%, respectively; P = 0.83).
CONCLUSIONS
The trial showed similar safety outcomes and no significant difference in functional independence with endovascular therapy after intravenous t-PA, as compared with intravenous t-PA alone. (Funded by the National Institutes of Health and others; ClinicalTrials.gov number, NCT00359424.)
Intravenous tissue plasminogen activator (t-PA; alteplase [Activase, Genentech, or Actilyse, Boehringer Ingelheim]) is the only proven reperfusion therapy for acute ischemic stroke, and its clinical effectiveness is critically time-dependent.1,2 A key advantage of intravenous t-PA is that it can be started rapidly after clinical assessment and computed tomography (CT) of the brain without the use of contrast material. However, few patients with ischemic stroke (<10%) meet current eligibility criteria for the use of intravenous t-PA, including arrival within a relatively short therapeutic time window (<4.5 hours) after symptom onset.1,3 Limitations of intravenous t-PA include dependence on available serum plasminogen, the resistance of an old or large thrombus to fibrinolysis, and the risks of systemic and cerebral hemorrhage.1,2,4,5
Endovascular therapy recanalizes occlusions in large arteries more frequently and rapidly than intravenous t-PA in patients with acute ischemic stroke and is increasingly used to treat patients with occlusions of the large intracranial arteries in institutions with the required expertise.6 Current endovascular approaches include endovascular pharmacologic thrombolysis, manipulation of the clot with the use of a guidewire or microcatheter, mechanical and aspiration thrombectomy, and most recently, stent-retriever technology. The primary disadvantage of endovascular therapy is the delay in initiation of treatment because of the time required to mobilize the interventional team and, in many cases, the need to transfer the patient to another hospital.7,8 Other potential limitations include difficulty getting the catheter to the site of occlusion, damage to the arterial wall from devices, fragmentation and distal embolization of the thrombus, risks associated with general anesthesia (if used), and complications of systemic and cerebral hemorrhage.7,9,10 In the absence of data from a randomized trial, it is uncertain whether endovascular therapy, with or without the previous use of intravenous t-PA, is more effective than intravenous t-PA alone.
Intravenous t-PA followed by endovascular therapy combines the advantages of a rapid start of intravenous t-PA with a greater likelihood of early recanalization with the use of endovascular therapy in patients with persistent occlusion after treatment with intravenous t-PA. On the basis of preliminary work, first tested in the small, randomized Emergency Management of Stroke (EMS) trial during 1995 and 199611 and consecutive single-group trials (the Interventional Management of Stroke [IMS] I and II trials),12,13 as well as the expanded clinical use of endovascular therapy after intravenous t-PA, the IMS III trial was organized to begin enrollment in 2006. In April 2012, after 656 of a planned 900 participants had undergone randomization, the data and safety monitoring board recommended to the sponsor (the National Institute of Neurological Disorders and Stroke) that enrollment be terminated owing to the crossing of the prespecified boundary for futility. Here we report the results of the prespecified primary efficacy and subgroup analyses and safety data through 90 days of follow-up.
METHODS
TRIAL DESIGN
We conducted the IMS III trial, an international, phase 3, randomized, open-label clinical trial with a blinded outcome, to test the approach of intravenous t-PA followed by protocol-approved endovascular treatment, as compared with standard intravenous t-PA. Intravenous t-PA was started within 3 hours after symptom onset in both groups. Details of the methods used in the trial have been published previously.14 The study protocol is available with the full text of this article at NEJM.org.
The design, analysis, and data collection for the IMS III trial, as well as the writing of the manuscript, were performed by members of the executive committee and investigators at the study sites (see the Supplementary Appendix, available at NEJM.org). These investigators vouch for the accuracy and completeness of the presented data and for the fidelity of this report to the study protocol. Genentech supplied t-PA for endovascular use, and EKOS, Concentric Medical, and Cordis Neurovascular supplied catheters; Genentech, EKOS, and Boehringer Ingelheim provided support for investigator meetings. None of the industry sponsors were involved in the study design, study conduct, manuscript review, or protocol review, except to make sure that the specified use of devices in the study followed the instructions for use approved by the Food and Drug Administration (FDA).
At the beginning of the trial, only a single thrombectomy device had been cleared for use by the FDA,15,16 and the trial leadership recognized that endovascular technology would continue to evolve. To keep the trial clinically relevant and optimize the endovascular approach, additional devices were allowed as they became cleared for clinical use by the regulatory authorities of participating countries, after approval by the executive committee of the IMS III trial.
At the beginning of the trial, CT angiography was used infrequently at participating hospitals to assess the presence of vascular occlusions in patients with acute stroke. Thus, the baseline National Institutes of Health Stroke Scale (NIHSS) score, a clinical measure of neurologic deficit with a range of 0 (no deficit) to 42 (maximum possible deficit), was used to identify patients with a score of 10 or more, who have a greater than 80% likelihood of a major arterial occlusion on subsequent angiography after intravenous t-PA.11,17,18 In amendment 3 to the protocol, after 284 participants had undergone randomization, identification of occlusion with the use of CT angiography was allowed to determine trial eligibility for patients with an NIHSS score of 8 or 9, because the routine use of CT angiography had increased rapidly during the early course of the study.19
To ensure that a similar, standard, FDA- approved total dose of t-PA (0.9 mg per kilogram of body weight administered over a 1-hour period; maximum dose, 90 mg) would be administered in patients assigned to endovascular therapy and those assigned to intravenous t-PA, the patients in the endovascular-therapy groups in the EMS and all IMS trials received only approximately two thirds of the standard dose of intravenous t-PA. Safety data on the standard dose of intravenous t-PA followed by additional intraarterial t-PA became available during the latter part of the IMS III trial, by which time this approach had become more common in clinical practice.20 Thus, the standard dose of intravenous t-PA was implemented in the endovascular-therapy group after the approval of amendment 5 to the protocol in June 2011.
PARTICIPANTS
We planned to enroll a maximum of 900 participants, 18 to 82 years of age, at 58 centers in the United States, Canada, Australia, and Europe. Eligibility criteria included receipt of intravenous t-PA within 3 hours after symptom onset and a moderate-to-severe neurologic deficit (defined as an NIHSS score ≥10 or, after approval of amendment 3, a score of 8 to 9 with CT angiographic evidence of an occlusion of the first segment of the middle cerebral artery [M1], internal carotid artery, or basilar artery at institutions where CT angiographic imaging at baseline was the standard of care for patients with acute stroke). Written informed consent was obtained from the patient or a legal representative before enrollment. Detailed inclusion and exclusion criteria are provided in Table 1 in the Supplementary Appendix.
TREATMENTS
All participants began receiving a standard dose of intravenous t-PA (0.9 mg per kilogram), with 10% as a bolus and the remainder infused over a 1-hour period (maximum dose, 90 mg). Throughout the trial, randomization was required within 40 minutes after the initiation of the infusion. The patients randomly assigned to the intravenous t-PA group received the remainder of the standard dose.
Participants randomly assigned to the endovascular-therapy group underwent angiography as soon as possible, either at the hospital that initiated treatment with intravenous t-PA or at another participating hospital. Participants who had no angiographic evidence of a treatable occlusion received no additional treatment, and those with a treatable vascular occlusion received endovascular intervention with an approach chosen by the site neurointerventionalist (i.e., thrombectomy with the Merci retriever [Concentric Medical], Penumbra System [Penumbra], or Solitaire FR revascularization device [Covidien], or endovascular delivery of t-PA by means of the Micro-Sonic SV infusion system [EKOS] or a standard microcatheter). The angiographic procedure had to begin within 5 hours and be completed within 7 hours after the onset of stroke. Heparin infusion was started intravenously with a 2000-unit bolus, followed by an infusion of 450 units per hour during endovascular therapy, and was discontinued at the end of the procedure.
CLINICAL ASSESSMENTS AND OUTCOMES
The primary outcome measure was a modified Rankin scale score of 2 or less (indicating functional independence) at 90 days. The modified Rankin score is a measure of disability and functional status after stroke that ranges from 0 (no symptoms) to 5 (severe disability and bedridden) and 6 (death).21 All modified Rankin scale assessments at 90 days were to be performed by study investigators who were not involved in the treatment of the patient and who were unaware of the treatment assignment. The patient’s functional status before the qualifying stroke was assessed by means of a modified Rankin score already documented in the patient’s medical history.
CT was performed at baseline, at 24 hours (±6 hours), and if there was a neurologic decline. CT angiography was performed at baseline at those study sites that routinely included it in their baseline imaging protocol. CT angiography was planned for all participants at 24 hours to assess vascular patency. The Thrombolysis in Cerebral Infarction (TICI) score, which ranges from 0 (no reperfusion) to 3 (full reperfusion in the distribution of the occluded artery), was used to assess the angiographic outcome in the endovascular-therapy group, for both recanalization of the original primary occlusive lesion and reperfusion of the distal vasculature of the occluded artery on completion of the angiographic procedure (see Table 4 in the Supplementary Appendix for further descriptions).22
STATISTICAL ANALYSIS
Participants were randomly assigned in a 2:1 ratio to endovascular therapy or intravenous t-PA alone with the use of an Internet-based, computerized algorithm of minimization and the biased-coin method, which accounted for two factors: clinical center and baseline NIHSS strata (scores of 8 to 19 vs. ≥20).23 We calculated that a sample of 900 patients would provide an effect size of 10 percentage points (the absolute difference between the endovascular-therapy and intravenous t-PA groups in the proportion of participants with a modified Rankin score of ≤2 at 90 days), assuming that 40% of the patients had a good outcome in the intravenous t-PA group, as noted in those patients in the NINDS rt-PA Stroke Study who had age and baseline stroke severity similar to the eligibility criteria for the IMS III trial1,12; type 1 and type 2 error probabilities of 0.05 (two-sided) and 0.20, respectively; an inflation factor of 1.03 to account for a noncompliance rate of approximately 2%; and the O’Brien and Fleming–type alpha-spending function24 for three interim efficacy analyses.
The prespecified criterion for futility was based on conditional power of less than 20% under the alternative hypothesis. The primary efficacy hypothesis was assessed with the use of the Cochran–Mantel–Haenszel test, with adjustment for the dichotomized baseline NIHSS score, and the weights of the Cochran–Mantel–Haenszel test were applied in the estimation of the risk difference.25 At both the interim and the final analyses, an unfavorable outcome (defined as a modified Rankin score of >2) was imputed for participants who had missing data for the primary outcome or for whom data on the primary outcome were obtained outside the specified window. For all analyses of predefined secondary outcomes and subgroup and safety analyses, each test was conducted at a two-sided alpha level of 0.01. Prespecified subgroup analyses included NIHSS strata, time from symptom onset to treatment (intravenous t-PA and endovascular therapy), presence or absence of arterial occlusion on CT angiography at baseline, age, sex, and presence or absence of atrial fibrillation. For the analysis of raw modified Rankin scores, we used the van Elteren test.26
RESULTS
CHARACTERISTICS OF THE PARTICIPANTS
A total of 656 participants underwent randomization (434 participants to endovascular therapy and 222 to intravenous t-PA alone) at 58 study centers between August 25, 2006, and April 17, 2012 in the United States (41 sites), Canada (7), Australia (4), and Europe (6) (see the Supplementary Appendix). Table 2 in the Supplementary Appendix lists reasons why screened patients did not undergo randomization, and Figure 1 in the Supplementary Appendix shows the numbers of patients who underwent study interventions. An unfavorable imputation was applied for 27 participants (14 participants for whom the primary outcome was assessed outside the specified 30-day window and 13 for whom the primary outcome was not assessed).
The only baseline variable that differed significantly between the two treatment groups was the proportion of patients with a history of coronary artery disease (P = 0.01) (Table 1). Data on the presence or absence of major arterial occlusions according to the NIHSS score for the 306 participants who underwent CT angiography at baseline are shown in Figure 2 in the Supplementary Appendix.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Endovascular Therapy (N = 434) | Intravenous t-PA Alone (N = 222) |
|---|---|---|
| Age — yr | ||
| Median | 69 | 68 |
| Range | 23–89 | 23–84 |
| Male sex — no. (%) | 218 (50.2) | 122 (55.0) |
| Race or ethnic group — no. (%)† | ||
| Black | 51 (11.8) | 19 (8.6) |
| Hispanic | 11 (2.5) | 12 (5.4) |
| NIHSS score‡ | ||
| Median | 17 | 16 |
| Range | 7–40 | 8–30 |
| ASPECTS of 8, 9, or 10 — no. (%)§ | 247 (56.9) | 131 (59.0) |
| Presumptive location of stroke — no. (%) | ||
| Left hemisphere | 224 (51.6) | 106 (47.7) |
| Right hemisphere | 197 (45.4) | 109 (49.1) |
| Brain stem or cerebellum | 10 (2.3) | 4 (1.8) |
| Unknown or multiple locations | 3 (0.7) | 3 (1.4) |
| Atrial fibrillation — no. (%) | 153 (35.3) | 70 (31.5) |
| History of hypertension — no. (%) | 319 (73.5) | 171 (77.0) |
| History of diabetes — no. (%) | 94 (21.7) | 54 (24.3) |
| History of congestive heart failure — no. (%) | 50 (11.5) | 31 (14.0) |
| History of coronary artery disease — no. (%) | 102 (23.5) | 72 (32.4) |
| History of hyperlipidemia — no. (%) | 215 (49.5) | 112 (50.5) |
| Serum glucose — mmol/liter | 7.4±2.9 | 7.6±3.1 |
| Time from stroke onset to initiation of intravenous t-PA — min | 122.4±33.7 | 121.2±33.8 |
| Modified Rankin scale score — no. (%)¶ | ||
| 0 | 379 (87.3) | 197 (88.7) |
| 1 | 35 (8.1) | 21 (9.5) |
| 2 | 19 (4.4) | 4 (1.8) |
| 3 | 1 (0.2) | 0 |
| Systolic blood pressure — mm Hg | 148±21.3 | 147.3±24 |
| Current antiplatelet use — no. (%) | 186 (42.9) | 108 (48.6) |
| Current statin use — no. (%) | 155 (35.7) | 83 (37.4) |
| International normalized ratio | ||
| Median | 1.0 | 1.0 |
| Range | 0.9–1.7 | 0.9–1.7 |
Plus–minus values are means ±SD. There were no significant between-group differences, except for history of coronary artery disease (P = 0.01). The abbreviation t-PA denotes tissue plasminogen activator.
Race or ethnic group was self-reported.
The National Institutes of Health Stroke Scale (NIHSS), a serial measure of neurologic deficit, is a 42-point scale that quantifies neurologic deficits in 11 categories, with 0 indicating normal function without neurologic deficit and higher scores indicating greater severity of deficit.
The Alberta Stroke Program Early Computed Tomography Score (ASPECTS) allows for the systematic assessment of 10 regions of the brain with the use of computed tomography, with a score of 1 indicating a normal region and 0 indicating a region showing signs of ischemia; total scores range from 10 (no evidence of early ischemia) to 0 (all 10 regions in the hemisphere show early ischemic changes).
Scores on the modified Rankin scale range from 0 to 6, with 0 indicating no symptoms, 1 no substantial disability despite the presence of symptoms, 2 slight disability, and 3 moderate disability necessitating some help; a score of 6 indicates death. Persons with a score of 0, 1, or 2 are considered to be functionally independent.
PRIMARY OUTCOME
The trial was stopped early because of futility, according to the prespecified rule. There was no significant difference between the endovascular-therapy and intravenous t-PA groups in the overall proportion of participants with a modified Rankin score of 2 or less (40.8% and 38.7%, respectively; absolute adjusted difference, 1.5 percentage points; 95% confidence interval [CI], −6.1 to 9.1, with adjustment for NIHSS strata) (Fig. 1). There was also no significant difference in the predefined subgroups of patients with an NIHSS score of 20 or more, indicating severe stroke (difference of 6.8 percentage points in favor of the endovascular-therapy group; 95% CI, −4.4 to 18.1), and patients with a score of 8 to 19, indicating moderately severe stroke (difference of −1.0 percentage point in favor of the intravenous t-PA group; 95% CI, −10.8 to 8.8) (Fig. 2).
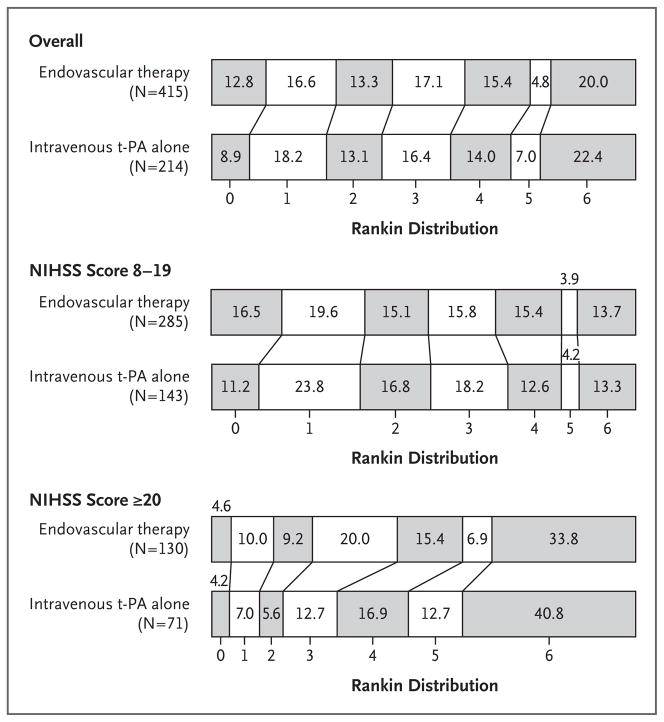
Figure 1. Distribution of Modified Rankin Scores, According to Study Group and Score on the National Institutes of Health Stroke Scale (NIHSS).
The percentages of patients are shown in or above each cell, according to score on the modified Rankin scale. Scores range from 0 to 6, with 0 indicating no symptoms, 1 no clinically significant disability (able to carry out all usual activities, despite some symptoms), 2 slight disability (able to look after own affairs without assistance but unable to carry out all previous activities), 3 moderate disability (requires some help but able to walk unassisted), 4 moderately severe disability (unable to attend to bodily needs without assistance and unable to walk unassisted), 5 severe disability (requires constant nursing care and attention, bedridden, and incontinent), and 6 death. Persons with a score of 0, 1, or 2 are considered to be functionally independent. Prespecified secondary analyses showed no significant differences between the two treatment groups across the entire distribution of the modified Rankin score overall (P = 0.25); among patients with an NIHSS score of 8 to 19, indicating moderately severe stroke (P = 0.83); or among those with an NIHSS score of 20 or more, indicating severe stroke (P = 0.06). The abbreviation t-PA denotes tissue plasminogen activator.
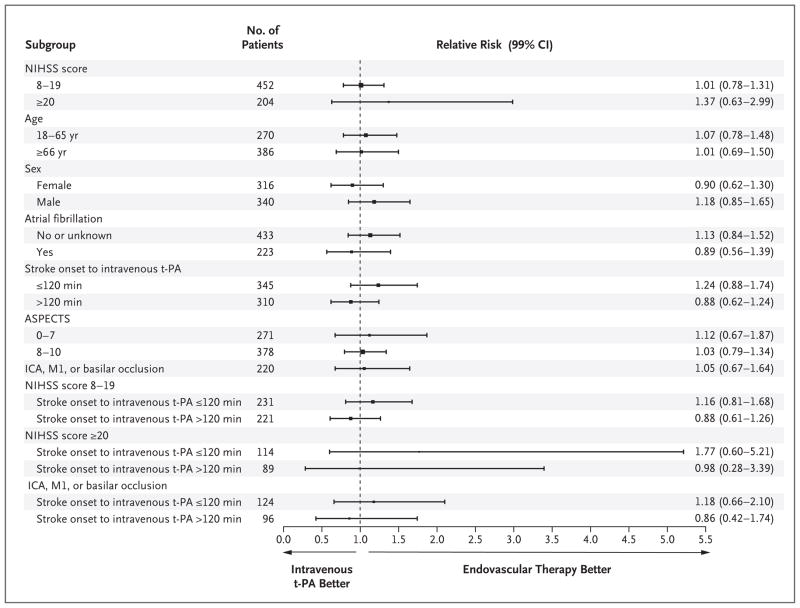
Figure 2. Adjusted Relative Risk for Predefined Subgroups, as Assessed According to the Primary Outcome of a Modified Rankin Score of 0 to 2 at 90 Days.
Data were adjusted for age (continuous), baseline NIHSS strata, and time from onset to initiation of intravenous t-PA (continuous). The comparisons of baseline NIHSS strata were not adjusted for baseline NIHSS score, and the subgroups defined according to the baseline NIHSS strata and time from onset to intravenous t-PA were adjusted only for age. One patient who underwent randomization did not receive intravenous t-PA but was included in the intention-to-treat analysis. The Alberta Stroke Program Early Computed Tomography Score (ASPECTS) allows for the systematic assessment of 10 regions of the brain with the use of computed tomography (CT), with a score of 1 indicating a normal region and 0 indicating a region showing signs of ischemia; total scores range from 10 (no evidence of early ischemia) to 0 (all 10 regions in the hemisphere show early ischemic changes). Data on ASPECTS were obtained for patients who had original CT scans for comparison. A total of 220 participants had an occlusion of the internal carotid artery (ICA), middle cerebral artery (M1), or basilar artery, as determined by means of CT angiography prior to treatment with intravenous t-PA.
SECONDARY OUTCOMES
Predefined secondary analyses showed no significant differences among the subgroups. The direction of effect favored better overall outcomes among participants in the endovascular-therapy group treated with intravenous t-PA within 2 hours after the onset of symptoms, as compared with those treated with intravenous t-PA alone within 2 hours after onset (Fig. 2, and Fig. 3 in the Supplementary Appendix), but the difference was not significant. There was a similar direction of effect toward better outcomes with a time from the start of intravenous t-PA to groin puncture of 90 minutes or less in the endovascular-therapy group, as compared with a procedure-initiation time of more than 90 minutes, but the difference was also not significant. Table 5 in the Supplementary Appendix provides details regarding the dosing of t-PA and treatment times.
REPERFUSION RATES
Reperfusion rates at angiography in the endovascular-therapy group, as measured according to TICI grades 2 or 3 (indicating partial or complete re-perfusion), were 65% for occlusion in the internal carotid artery (65 patients), 81% for an M1 occlusion (135 patients), 70% for a single occlusion in the second division of the middle cerebral artery (M2) (61 patients), and 77% for multiple M2 occlusions (22 patients). Only 4 patients had basilar occlusions, and the TICI score was not used for this location. Data regarding results in other vessels with smaller numbers of patients are not shown. Reperfusion rates, as measured by a TICI score of 2b (partial reperfusion of half or more of the vascular distribution of the occluded artery) to 3, were 38% for an occlusion in the internal carotid artery, 44% for an occlusion in M1, 44% for a single M2 occlusion, and 23% for multiple M2 occlusions.
The proportion of patients with a modified Rankin score of 2 or less at 90 days (primary outcome) increased with greater reperfusion. The primary outcome occurred in 12.7% of the 55 patients with a TICI score of 0, in 27.6% of the 29 patients with a TICI score of 1, in 34.3% of the 108 patients with a TICI score of 2a (partial perfusion of less than half the vascular distribution of the occluded artery), in 47.9% of the 119 patients with a TICI score of 2b, and in 71.4% of the 7 patients with a TICI score of 3 (P<0.001). Among patients with an occlusion of the internal carotid artery, M1, or both, reperfusion rates, as measured by a TICI score of 2 to 3, according to the various endovascular approaches, were 71% for intraarterial t-PA (51 patients), 71% for the MicroSonic SV infusion system with intraarterial t-PA (14 patients), 73% for the Merci retriever (77 patients), 85% for the Penumbra System (39 patients), and 75% for the Solitaire FR revascularization device (4 patients).
Among the 147 participants in the endovascular-therapy group for whom CT angiograms were obtained at both baseline and 24 hours, the rate of partial or complete recanalization at 24 hours was 81% for an occlusion in the internal carotid artery, 86% for an M1 occlusion, 88% for an M2 occlusion, and 100% for a basilar occlusion (only 1 patient had basilar occlusions). The rates in the intravenous t-PA group, among 69 patients with both sets of CT angiograms, were 35% for an occlusion in the internal carotid artery, 68% for an M1 occlusion, and 77% for an M2 occlusion.
SAFETY
Table 2 lists the predefined safety outcomes. There were no significant differences in mortality at 7 days or 90 days, in the rate of symptomatic intracerebral hemorrhage, or in the rate of parenchymal hematoma, although the rate of asymptomatic intracerebral hemorrhage was higher in the endovascular-therapy group than in the intravenous t-PA group (P = 0.01) (see Table 6 in the Supplementary Appendix for a list of all serious adverse events).
Table 2.
Primary and Secondary Safety End Points.*
| End Point | Endovascular Therapy (N = 434) | Intravenous t-PA Alone (N = 222) | P Value |
|---|---|---|---|
| Death — no. (%) | |||
| Within 7 days | 52 (12.0) | 24 (10.8) | 0.57 |
| Within 90 days | 83 (19.1) | 48 (21.6) | 0.52 |
| Intracerebral hemorrhage within 30 hr — no. (%) | |||
| Symptomatic | 27 (6.2) | 13 (5.9) | 0.83 |
| Asymptomatic | 119 (27.4) | 42 (18.9) | 0.01 |
| Parenchymal hematoma identified within 30 hr — no./total no. (%)† | |||
| Type 2 | 25/417 (6.0) | 13/207 (6.3) | 0.90 |
| Type 1 | 15/417 (3.6) | 3/207 (1.4) | 0.12 |
| Hemorrhage — no./total no. (%) | |||
| Subarachnoid | 48/417 (11.5) | 12/207 (5.8) | 0.02 |
| Intraventricular | 27/417 (6.5) | 10/207 (4.8) | 0.40 |
| Major complication due to nonintracerebral bleeding within 5 days — no. (%)‡ | 13 (3.0) | 5 (2.3) | 0.55 |
| Recurrent stroke within 90 days — no. (%) | 22 (5.1) | 14 (6.3) | 0.54 |
| Device or procedural complication — no. (%)‡ | 70 (16.1) | — | |
Events occurred during specified periods after the administration of intravenous t-PA. P values were obtained with the use of the Cochran–Mantel–Haenszel test. Data for events identified with the use of computed tomography exclude 32 participants for whom a scan was not obtained within 24 hours after initiation of intravenous t-PA or a postbaseline safety scan was not obtained within the defined time window (i.e., participants who died, had care withdrawn at the request of the family, or underwent imaging after the 30-hour window).
Parenchymal hematoma type 2 was defined as a dense hematoma involving more than 30% of the infarcted area with substantial space-occupying effect or any hemorrhagic area outside the infarcted area, and type 1 as a hematoma involving 30% or less of the infarcted area.
Complications included groin hematoma, vessel dissection, vessel perforation, and emboli in a previously uninvolved territory, as identified by the site investigator or as assessed centrally.
DISCUSSION
The IMS III trial was stopped early because of futility, according to the prespecified rules, and failed to show a benefit in functional outcome with the use of additional endovascular therapy, as compared with the standard therapy of intravenous t-PA alone. The safety profiles were similar in the two treatment groups.
We designed a stratified analysis for the primary outcome, hypothesizing that the efficacy of endovascular therapy would be greater in participants with more severe stroke (NIHSS score ≥20), since such patients have the highest likelihood of occlusion in a major intracranial artery and the greatest volume of ischemic brain at risk.12–14 In this subgroup, the difference in the proportion of participants with a modified Rankin score of 2 or less at 90 days in the endovascular-therapy group, as compared with those treated with intravenous t-PA alone, was not significant (6.8 percentage points; 95% CI, −4.4 to 18.1), and a larger difference among patients with more severe deficits who were treated within 2 hours after the onset of stroke was also not significant (14.0 percentage points; 99% CI, −6.2 to 34.1).
Although an earlier time to endovascular therapy was hypothesized to be associated with greater benefit, the results of relevant prespecified subgroup analyses were not significant. Trials of acute myocardial infarction have shown increased efficacy of percutaneous coronary intervention, as compared with fibrin-specific thrombolysis (1 percentage point lower mortality with percutaneous coronary intervention); efficacy is strongly related to both the rapidity of initiation of treatment and the severity and extent of myocardial ischemia.27,28 Given these data from trials of reperfusion in patients with myocardial infarction, the strong relationship between the time from symptom onset to the initiation of treatment and the clinical effectiveness of intravenous t-PA, and subgroup data from the IMS III trial, future trials of endovascular therapy should consider methods to minimize delays to the initiation of endovascular therapy. In addition, although we did not find a significant benefit of endovascular therapy in patients with severe stroke or occlusion of a large artery, a larger trial that is sufficiently powered to assess these subgroups might show efficacy.
Although successful revascularization in the IMS III trial was associated with better functional outcomes in the endovascular-therapy group, there are limitations of revascularization as a surrogate measure for differential efficacy between the two reperfusion therapies. In this trial, we observed partial or complete reperfusion in 81% of M1 occlusions, as compared with a reported rate of 40% recanalization for M1 occlusions as measured by means of transcranial Doppler ultrasonography and magnetic resonance angiography 2 to 3 hours after treatment with intravenous t-PA alone.7,29,30 Thus, although the endovascular approach provides an estimated increase of 40 percentage points in revascularization after the procedure, as compared with intravenous t-PA alone, we observed no significant clinical benefit of endovascular therapy after intravenous t-PA.
The single-group IMS I and II trials and the RECANALISE study indicate that the link between reperfusion and outcome is rapidly attenuated with increasing time from the onset of symptoms to reperfusion; in the IMS I and II trials, a 30-minute delay was associated with a 10% decrease in the probability of functional independence (defined as a modified Rankin score of 0, 1, or 2).31,32 Despite a strong emphasis on rapid treatment, the time to endovascular treatment in the IMS III trial was 32 minutes longer than in the IMS I trial, which was a smaller, phase 2, single-group study conducted at 17 sites. This may be one important reason for the lack of clinical benefit, despite the finding of substantially better revascularization with endovascular therapy than with intravenous t-PA.
Two recent phase 2 trials that compared stent retrievers with the Merci retriever showed clear and substantial increases in reperfusion in favor of the stent retrievers.33,34 Stent retrievers were used in only a small number of patients in the IMS III trial before the study was halted because of futility. Hence, one limitation of our trial is that it did not compare the efficacy of the new stent retrievers with that of intravenous t-PA alone. However, our study highlights the finding that improved reperfusion is not a guarantee of clinical efficacy. The efficacy of these new devices, as compared with intravenous t-PA alone, remains to be demonstrated.
The IMS III trial and other recent trials of endovascular therapy for acute ischemic stroke address the promise and limitations of endovascular therapy. The use of randomization in ongoing and future stroke trials, rather than the treatment of eligible patients with endovascular therapy outside any trial, and minimization of the time to treatment will be essential for assessing the potential benefit of endovascular therapy for acute ischemic stroke.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health and the National Institute of Neurological Disorders and Stroke (UC U01NS052220, MUSC U01NS054630, and U01NS077304) and by Genentech, EKOS, Concentric Medical, Cordis Neurovascular, and Boehringer Ingelheim.
Dr. Broderick reports receiving consulting fees from Photo-Thera, receiving lecture fees from Oakstone Publishing, and receiving a drug from Schering-Plough for use in a study funded by the National Institutes of Health. Dr. Palesch reports receiving consulting fees from BrainsGate and Edge Therapeutics. Dr. Demchuk reports receiving grant support and lecture fees from Covidien. Dr. Khatri reports receiving consulting fees from Penumbra, Genentech, and Janssen Pharmaceuticals; providing expert testimony for Medico-Legal Consulting; receiving lecture fees from Medical Dialogues; receiving royalties from Informa, and receiving travel support from Genentech. Dr. Hill reports receiving consulting fees from Vernalis Group; receiving grant support from Hoffmann–La Roche Canada; receiving lecture fees from Hoffmann–La Roche Canada, Servier Canada, and Bristol-Myers Squibb Canada; receiving stock options from Calgary Scientific; receiving support from the Heart and Stroke Foundation of Alberta, Northwest Territories, and Nunavut and Alberta Innovates–Health Solutions; and serving as an unpaid board member for the Heart and Stroke Foundation of Alberta, Northwest Territories, and Nunavut and the Institute of Circulatory and Respiratory Health of the Canadian Institutes of Health Research. Dr. Jovin reports receiving consulting fees and stock options from Silk Road Medical. Dr. Silver reports serving as an unpaid board member for Boehringer Ingelheim Canada, Bayer Canada, and Bristol-Myers Squibb and Pfizer Canada; receiving lecture fees from Boehringer Ingelheim Canada and Servier Canada; and receiving travel support from Boehringer Ingelheim Canada. Dr. von Kummer reports serving as a board member for Lundbeck and Boehringer Ingelheim; receiving consulting fees from Lundbeck; and receiving lecture fees from Penumbra, Boehringer Ingelheim, and Lundbeck. Dr. Demaerschalk reports receiving grant support and consultant fees from Genentech. Dr. Budzik reports receiving consulting and lecture fees from Concentric Medical. Dr. Goyal reports receiving consulting and lecture fees from Covidien; receiving grant support from Covidien; and owning stock options in Calgary Scientific and NoNO. Dr. Engelter reports serving as a board member for Boehringer Ingelheim and receiving travel support from Boehringer Ingelheim. Dr. Anderson reports receiving lecture fees and travel support from GE Healthcare. Ms. Spilker reports receiving payment for the development of educational presentations from The Stroke Group and serving as a board member for The Stroke Interventionalist: The Clinical Journal of Acute Stroke Treatment. Dr. Tomsick reports receiving grant support from Covidien.
APPENDIX
The authors’ affiliations are as follows: the Departments of Neurology and Rehabilitation Medicine and Radiology, University of Cincinnati Neuroscience Institute, University of Cincinnati Academic Health Center, Cincinnati (J.P.B., P.K., J.S., J.C., T.A.T.); the Department of Public Health Sciences (Y.Y.P, S.D.Y., R.L.M., L.D.F.) and the Division of Emergency Medicine (E.C.J.), Medical University of South Carolina, Charleston; the Seaman Family Magnetic Resonance Research Centre, Departments of Clinical Neurosciences and Radiology, Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada (A.M.D., M.D.H., M.G., K.J.R.); the Stroke Institute, University of Pittsburgh Medical Center, Pittsburgh (T.G.J.); Melbourne Brain Centre, Royal Melbourne Hospital, University of Melbourne, Melbourne, VIC, Australia (B.Y.); the University of Toronto, Department of Medicine, Division of Neurology and the University Health Network, Toronto (F.L.S.); the Department of Neuroradiology, Dresden University Stroke Center, University Hospital, Dresden, Germany (R.K.); the Neurovascular Unit, Department of Neurology, Hospital Universitari Vall d’Hebron, Barcelona (C.A.M.); the Department of Neurology, Mayo Clinic, Phoenix, AZ (B.M.D.); the OhioHealth Neuroscience Institute, Riverside Methodist Hospital, Columbus, OH (R.B.); Oregon Stroke Center, Oregon Health and Science University, Portland (W.M.C.); the Department of Radiology, Medical College of Wisconsin, Milwaukee (O.O.Z.); Alexian Brothers Medical Center, Elk Grove Village, IL (T.W.M.); the Department of Neurology, University Medical Center Utrecht and the Rudolph Magnus Institute of Neurosciences, Utrecht, and the St. Antonius Hospital, Nieuwegein — both in the Netherlands (W.J.S.); the Department of Neurology and Stroke Center, Bichat University Hospital, Paris (M.M.); the Department of Neurology, Basel University Hospital, Basel, Switzerland (S.T.E.); the George Institute for Global Health, Royal Prince Alfred Hospital, University of Sydney, Sydney (C.A.); and the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD (L.S.J.).
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 3.de Los Ríos la Rosa F, Khoury J, Kissela BM, et al. Eligibility for intravenous recombinant tissue-type plasminogen activator within a population: the effect of the European Cooperative Acute Stroke Study (ECASS) III Trial. Stroke. 2012;43:1591–5. doi: 10.1161/STROKEAHA.111.645986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riedel CH, Zimmermann P, Jensen-Kondering U, Stingele R, Deuschl G, Jansen O. The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke. 2011;42:1775–7. doi: 10.1161/STROKEAHA.110.609693. [DOI] [PubMed] [Google Scholar]
- 5.Hirano T, Sasaki M, Mori E, et al. Residual vessel length on magnetic resonance angiography identifies poor responders to alteplase in acute middle cerebral artery occlusion patients: exploratory analysis of the Japan Alteplase Clinical Trial II. Stroke. 2010;41:2828–33. doi: 10.1161/STROKEAHA.110.594333. [DOI] [PubMed] [Google Scholar]
- 6.Meyers PM, Schumacher HC, Connolly ES, Jr, Heyer EJ, Gray WA, Higishada RT. Current status of endovascular stroke treatment. Circulation. 2011;123:2591–601. doi: 10.1161/CIRCULATIONAHA.110.971564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomsick TA, Khatri P, Jovin T, et al. Equipoise among recanalization strategies. Neurology. 2010;74:1069–76. doi: 10.1212/WNL.0b013e3181d76b8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonarelli SB, Tibbs M, Vazquez G, Lakshminarayan K, Rodriguez GJ, Qureshi AI. Accuracy of the new ICD-9-CM code for “drip-and-ship” thrombolytic treatment in patients with ischemic stroke. J Stroke Cerebrovasc Dis. 2012;21:121–3. doi: 10.1016/j.jstrokecerebrovasdis.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Abou-Chebl A, Lin R, Hussain MS, et al. Conscious sedation versus general anesthesia during endovascular therapy for acute anterior circulation stroke: preliminary results from a retrospective, multi-center study. Stroke. 2010;41:1175–9. doi: 10.1161/STROKEAHA.109.574129. [DOI] [PubMed] [Google Scholar]
- 10.Khatri P, Wechsler LR, Broderick JP. Intracranial hemorrhage associated with revascularization therapies. Stroke. 2007;38:431–40. doi: 10.1161/01.STR.0000254524.23708.c9. [DOI] [PubMed] [Google Scholar]
- 11.Lewandowski CA, Frankel M, Tomsick TA, et al. Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: Emergency Management of Stroke (EMS) Bridging Trial. Stroke. 1999;30:2598–605. doi: 10.1161/01.str.30.12.2598. [DOI] [PubMed] [Google Scholar]
- 12.IMS Study Investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke. 2004;35:904–11. doi: 10.1161/01.STR.0000121641.77121.98. [DOI] [PubMed] [Google Scholar]
- 13.The IMS II Trial Investigators. The Interventional Management of Stroke (IMS) II Study. Stroke. 2007;38:2127–35. doi: 10.1161/STROKEAHA.107.483131. [DOI] [PubMed] [Google Scholar]
- 14.Khatri P, Hill M, Palesch Y, et al. Methodology of the Interventional Management of Stroke III Trial. Int J Stroke. 2008;3:130–7. doi: 10.1111/j.1747-4949.2008.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432–8. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 16.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205–12. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 17.Fischer U, Arnold M, Nedeltchev K, et al. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke. 2005;36:2121–5. doi: 10.1161/01.STR.0000182099.04994.fc. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima M, Kimura K, Ogata T, et al. Relationships between angiographic findings and National Institutes of Health stroke scale score in cases of hyperacute carotid ischemic stroke. AJNR Am J Neuroradiol. 2004;25:238–41. [PMC free article] [PubMed] [Google Scholar]
- 19.Mackey J, Khatri P, Broderick JP. Increasing use of CT angiography in interventional study sites: the IMS III experience. AJNR Am J Neuroradiol. 2010;31(3):E34. doi: 10.3174/ajnr.A1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaltoni HM, Albright KC, Gonzales NR, et al. Is intra-arterial thrombolysis safe after full-dose intravenous recombinant tissue plasminogen activator for acute ischemic stroke? Stroke. 2007;38:80–4. doi: 10.1161/01.STR.0000251720.25337.b0. [DOI] [PubMed] [Google Scholar]
- 21.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–7. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 22.Tomsick T. TIMI, TIBI, TICI: I came, I saw, I got confused. AJNR Am J Neuroradiol. 2007;28:382–4. [PMC free article] [PubMed] [Google Scholar]
- 23.Pocock S. Clinical trials — a practical approach. New York: Wiley; 1983. [Google Scholar]
- 24.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 25.Hall DB, Woolson RF, Clarke WR, et al. Handbook of statistics. Vol. 18. Bio-environmental and public health statistics. Amsterdam: North-Holland; 2000. Cochran-Mantel-Haenszel techniques: applications involving epidemiologic survey data; pp. 483–500. [Google Scholar]
- 26.van Elteren PH. On the combination of independent two-sample tests of Wilcoxon. Bull Int Stat Inst. 1960;37:351–61. [Google Scholar]
- 27.Boersma E. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J. 2006;27:779–88. doi: 10.1093/eurheartj/ehi810. [DOI] [PubMed] [Google Scholar]
- 28.De Luca G, Cassetti E, Marino P. Percutaneous coronary intervention-related time delay, patient’s risk profile, and survival benefits of primary angioplasty vs lytic therapy in ST-segment elevation myocardial infarction. Am J Emerg Med. 2009;27:712–9. doi: 10.1016/j.ajem.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Alexandrov AV, Demchuk AM, Burgin WS, Robinson DJ, Grotta JC. Ultrasound-enhanced thrombolysis for acute ischemic stroke: phase I — findings of the CLOTBUST trial. J Neuroimaging. 2004;14:113–7. [PubMed] [Google Scholar]
- 30.Marks MP, Olivot JM, Kemp S, et al. Patients with acute stroke treated with intravenous tPA 3–6 hours after stroke onset: correlations between MR angiography findings and perfusion- and diffusion-weighted imaging in the DEFUSE study. Radiology. 2008;249:614–23. doi: 10.1148/radiol.2492071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73:1066–72. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazighi M, Serfaty JM, Labreuche J, et al. Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol. 2009;8:802–9. doi: 10.1016/S1474-4422(09)70182-6. [DOI] [PubMed] [Google Scholar]
- 33.Saver JL, Jahan R, Levy E, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–9. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 34.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–40. doi: 10.1016/S0140-6736(12)61299-9. [Erratum, Lancet 2012; 380:1230.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.