Abstract
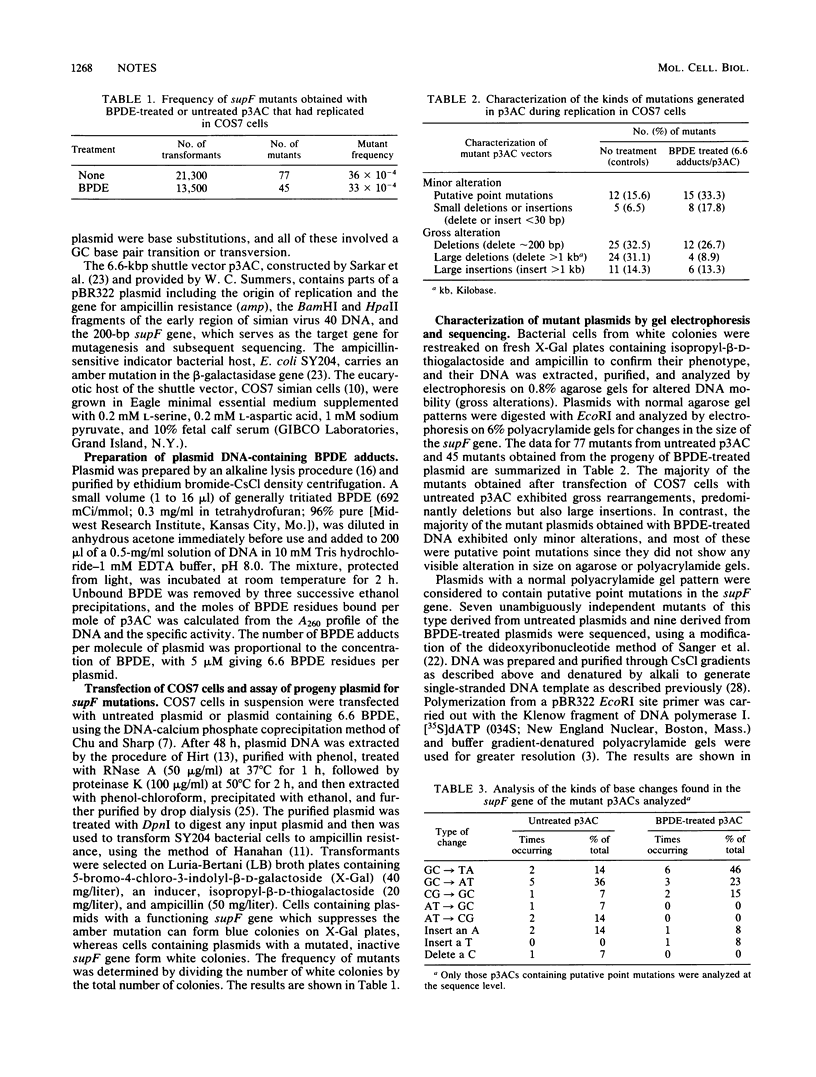
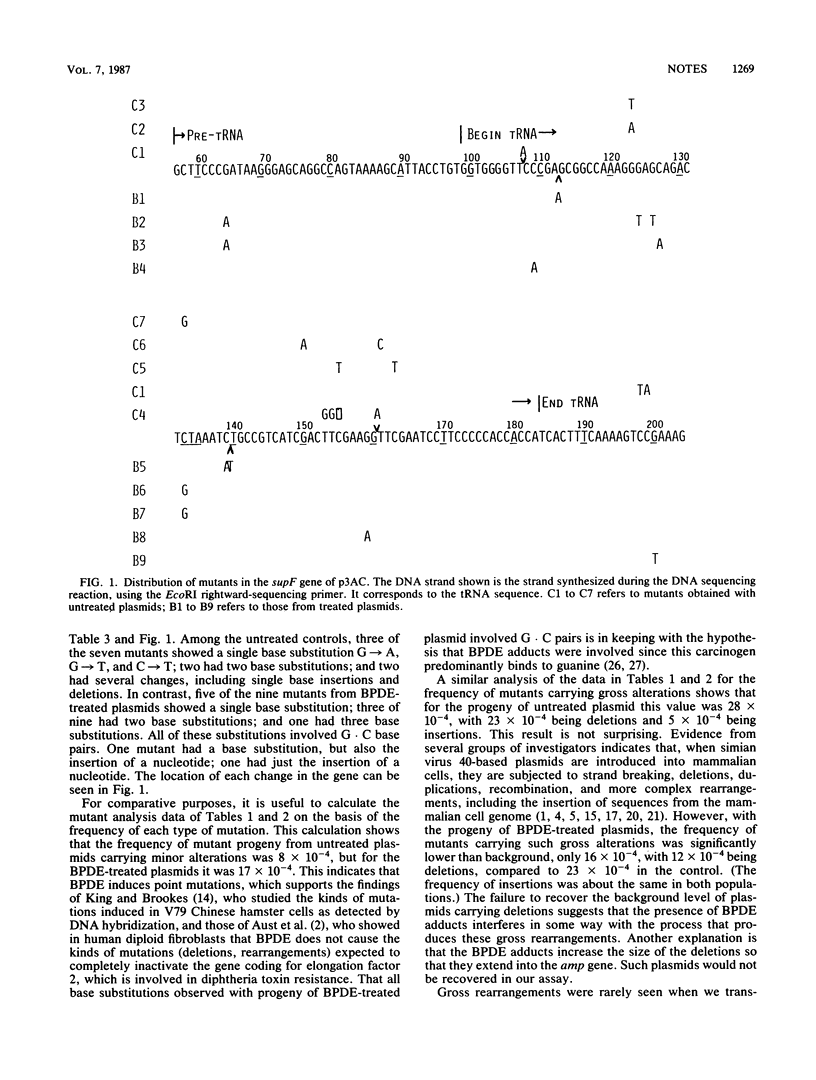
We have investigated the kinds of mutations induced when a shuttle vector containing covalently bound residues of the (+/-)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene (BPDE) replicates in the monkey kidney cell line COS7. The target for detecting mutations was the 200-base pair gene for a tyrosine suppressor tRNA (supF), inserted at the EcoRI site in shuttle vector p3AC (Sarkar et al., Mol. Cell. Biol. 4:2227-2230, 1984). When introduced by transformation, a functioning supF gene in progeny plasmid recovered from COS7 cells allows suppression of a lacZ amber mutation in the indicator Escherichia coli host. Treatment of p3AC with BPDE caused a linear increase in the number of BPDE residues bound per plasmid. Untreated plasmids and plasmids containing 6.6 BPDE residues were transfected into COS7 cells, and the progeny were assayed for mutations in the supF gene. The frequency of mutants generated during replication of the BPDE-treated plasmids was not higher than that from untreated plasmids, but the two populations differed markedly in the kinds of mutations they contained. Gel electrophoresis analysis of the size alterations of 77 mutant plasmids obtained with untreated DNA and 45 obtained with BPDE-treated DNA showed that the majority of the mutant progeny of untreated plasmids exhibited gross alterations, principally large deletions. In contrast, the majority of the mutants generated during replication of the BPDE-treated plasmids contained only minor alterations, principally point mutations. Sequence analysis of progeny of untreated plasmids containing putative point mutations showed insertions and deletions of bases and a broad spectrum of base substitutions; in those from BPDE-treated plasmids, all base substitutions involved guanosine . cystosine pairs.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman C. R., Davidson R. L. High spontaneous mutation frequency in shuttle vector sequences recovered from mammalian cellular DNA. Mol Cell Biol. 1984 Nov;4(11):2266–2272. doi: 10.1128/mcb.4.11.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aust A. E., Drinkwater N. R., Debien K., Maher V. M., McCormick J. J. Comparison of the frequency of diphtheria toxin and thioguanine resistance induced by a series of carcinogens to analyze their mutational specificities in diploid human fibroblasts. Mutat Res. 1984 Jan;125(1):95–104. doi: 10.1016/0027-5107(84)90036-8. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calos M. P., Lebkowski J. S., Botchan M. R. High mutation frequency in DNA transfected into mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3015–3019. doi: 10.1073/pnas.80.10.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Joffe S., Seidman M. M. Recombination and deletion of sequences in shuttle vector plasmids in mammalian cells. Mol Cell Biol. 1985 Sep;5(9):2265–2271. doi: 10.1128/mcb.5.9.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Mizusawa H., Seidman M. Survival and mutagenesis of bacterial plasmids with localized carcinogen adducts. Mutat Res. 1984 Apr;126(2):127–137. doi: 10.1016/0027-5107(84)90054-x. [DOI] [PubMed] [Google Scholar]
- Chu G., Sharp P. A. SV40 DNA transfection of cells in suspension: analysis of efficiency of transcription and translation of T-antigen. Gene. 1981 Mar;13(2):197–202. doi: 10.1016/0378-1119(81)90008-1. [DOI] [PubMed] [Google Scholar]
- Eisenstadt E., Warren A. J., Porter J., Atkins D., Miller J. H. Carcinogenic epoxides of benzo[a]pyrene and cyclopenta[cd]pyrene induce base substitutions via specific transversions. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1945–1949. doi: 10.1073/pnas.79.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer P. M., Sarkar S. N., Summers W. C. Detection and analysis of UV-induced mutations in mammalian cell DNA using a lambda phage shuttle vector. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1041–1044. doi: 10.1073/pnas.83.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hauser J., Seidman M. M., Sidur K., Dixon K. Sequence specificity of point mutations induced during passage of a UV-irradiated shuttle vector plasmid in monkey cells. Mol Cell Biol. 1986 Jan;6(1):277–285. doi: 10.1128/mcb.6.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- King H. W., Brookes P. On the nature of the mutations induced by the diolepoxide of benzo[a]pyrene in mammalian cells. Carcinogenesis. 1984 Jul;5(7):965–970. doi: 10.1093/carcin/5.7.965. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., DuBridge R. B., Antell E. A., Greisen K. S., Calos M. P. Transfected DNA is mutated in monkey, mouse, and human cells. Mol Cell Biol. 1984 Oct;4(10):1951–1960. doi: 10.1128/mcb.4.10.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Lebkowski J. S., Greisen K. S., Calos M. P. Specificity of mutations induced in transfected DNA by mammalian cells. EMBO J. 1984 Dec 20;3(13):3117–3121. doi: 10.1002/j.1460-2075.1984.tb02267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa H., Lee C. H., Kakefuda T. Alteration of plasmid DNA-mediated transformation and mutation induced by covalent binding of benzo[alpha]pyrene-7,8-dihydrodiol-9,10-oxide in Escherichia coli. Mutat Res. 1981 Jun;82(1):47–57. doi: 10.1016/0027-5107(81)90137-8. [DOI] [PubMed] [Google Scholar]
- Mizusawa H., Lee C. H., Kakefuda T., McKenney K., Shimatake H., Rosenberg M. Base insertion and deletion mutations induced in an Escherichia coli plasmid by benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6817–6820. doi: 10.1073/pnas.78.11.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque A., Chakrabarti S., Joffee S., Seidman M. Mutagenesis of a shuttle vector plasmid in mammalian cells. Mol Cell Biol. 1984 Mar;4(3):435–441. doi: 10.1128/mcb.4.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque A., Mizusawa H., Seidman M. M. Rearrangement and mutagenesis of a shuttle vector plasmid after passage in mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3010–3014. doi: 10.1073/pnas.80.10.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Dasgupta U. B., Summers W. C. Error-prone mutagenesis detected in mammalian cells by a shuttle vector containing the supF gene of Escherichia coli. Mol Cell Biol. 1984 Oct;4(10):2227–2230. doi: 10.1128/mcb.4.10.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman M. M., Dixon K., Razzaque A., Zagursky R. J., Berman M. L. A shuttle vector plasmid for studying carcinogen-induced point mutations in mammalian cells. Gene. 1985;38(1-3):233–237. doi: 10.1016/0378-1119(85)90222-7. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B., Jeffrey A. M., Jennette K. W., Blobstein S. H., Harvey R. G., Harris C., Autrup H., Kasai H., Nakanishi K. Benzo(a)pyrene diol epoxides as intermediates in nucleic acid binding in vitro and in vivo. Science. 1976 Aug 13;193(4253):592–595. doi: 10.1126/science.959820. [DOI] [PubMed] [Google Scholar]
- Yang L. L., Maher V. M., McCormick J. J. Error-free excision of the cytotoxic,mutagenic N2-deoxyguanosine DNA adduct formed in human fibroblasts by (+/-)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5933–5937. doi: 10.1073/pnas.77.10.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]


