Abstract
Autism is a common and often severe neurodevelopmental disorder for which diverse pathophysiological processes have been proposed. Recent gene expression data comparing autistic and control brains suggest that the normal differential gene expression between frontal and temporal cortex is attenuated in autistic brains. It is unknown if regional de-differentiation occurs elsewhere in autistic brain. Using high resolution, genome-wide RNA expression microarrays and brain specimens meeting stringent selection criteria we evaluated gene expression data of two other regions: Brodmann area 19 (occipital cortex) and cerebellar cortex. In contrast to frontal/temporal cortical data, our data do not indicate an attenuation of regional specialization between occipital and cerebellar cortical regions in autistic brains.
Autism is a common and often severe neurodevelopmental condition of high heritability1. Because of the personal, family and societal burden of autism, understanding the causes and pathophysiological basis of autism is a public health priority. More than 100 uncommon and rare monogenic and genomic copy number variants are known causes or major contributing factors of autism, but most cases are idiopathic2. Neuropathological, neuroimaging, neurophysiological, molecular genetic, biochemical and bioinformatics analyses have each provided data leading to the numerous hypotheses regarding underlying pathophysiological processes in autism3.Voineagu et al. recently made the important observation that regional patterns of gene expression that distinguish frontal and temporal cortex are significantly attenuated in autistic brain4. This, in turn, suggests an abnormality of patterning of cortical gene expression as a pathophysiological process in autism. It is unknown if regional de-differentiation occurs elsewhere in autistic brains.
We asked whether gene expression distinctions in other human brain regions, in particular, between the Brodmann area 19 (BA19) of the occipital cortex and the cerebellar cortex, are attenuated in autistic brain. We therefore compared gene expression profiles of these brain regions using specimens meeting stringent selection criteria that were evaluated with high resolution, genome-wide RNA expression microarrays. Earlier work demonstrated the validity of the quantitative gene expression data using this approach5.
Results
Our data do not provide evidence for attenuation of differences in regional differentiation between autistic and control brain in the regions that we evaluated. The number of genes that are differentially expressed between BA19 and cerebellum in control brain compared to autistic brain (ΔΔC-A) using a false discovery rate (FDR) of 1% and a log fold change (LFC) of 1 is 1.2 fold (Table 1). The ΔΔC-A increased as the LFC threshold was relaxed. This suggested that the seeming difference noted at low or absent LFC thresholds might be due to differences in gene expression variances between groups and, in particular, greater variances within the autism group, as suggested by our previous work5. Indeed, fold change values for differential regional expression in control brain are highly correlated to those in autistic brain, while p-values are not (Supplementary Fig. 1).
Table 1. Number of differentially expressed probes between cerebellar cortex and occipital cortex (BA19) at varying thresholds of false discovery rate and log fold change. FDR = False discovery rate; LFC = log fold change.
| FDR | ||||
|---|---|---|---|---|
| 5% | 1% | |||
| LFC | Autism | Control | Autism | Control |
| 0 | 5057 | 9845 | 1745 | 5998 |
| 1 | 1277 | 1294 | 1030 | 1280 |
| 1.5 | 528 | 473 | 479 | 473 |
| 2 | 213 | 207 | 203 | 207 |
Next, although quantitative differences in regional differentiation between autistic and control brain were not apparent, we explored qualitative differences in the probe lists using a bioinformatics approach. Using a FDR = 1% and LFC = 1 we explored those probes that are regionally differentially expressed in control vs. autistic brain (Fig. 1; Supplementary Table 1). The majority of differentially expressed probes between BA19 cortex and cerebellar cortex are shared in both control and autism brain. Moreover, even for those genes uniquely differentially expressed in the autism or control group, the biological functions of the genes are similar, and included genes associated with synapse development or synaptic function (Figure 2).
Figure 1. Differentially expressed RNAs between cerebellar cortex and occipital cortex (BA19) comparing autistic and control brain.
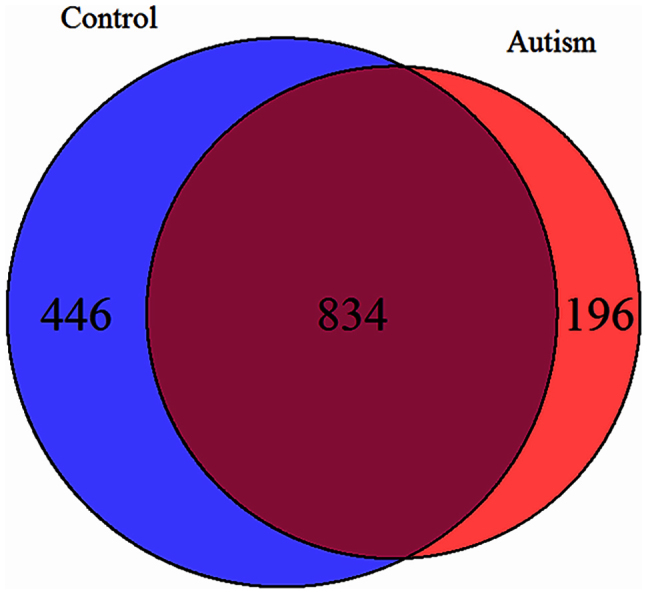
Differentially expressed probes common to both types of brains as well as those that are unique to one type or another are noted.
Figure 2. Gene ontology enrichment analysis for uniquely differentially expressed RNAs between cerebellar cortex and occipital cortex (BA19) in control brain (Panel A) and autistic brain (Panel C), and those differentially expressed RNAs that are shared in both autistic and control brain (Panel B).
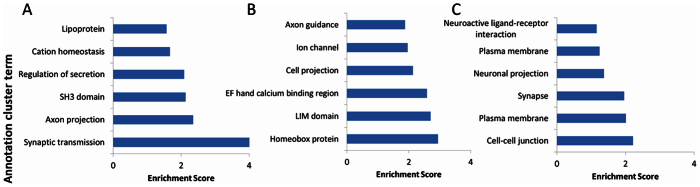
The enrichment score represents the negative logarithm of the p-value evaluating the significance of gene ontology terms for regionally differentially expressed RNAs. The top 6 annotation clusters are listed as derived from the DAVID bioinformatics tool.
Our analysis of the ΔΔC-A demonstrated sensitivity to LFC thresholds. Voineagu et al. reported differences in the differential gene expression between frontal and temporal cortex in control vs. autistic brain, but did not report the magnitude of the differential gene expression differences4. We therefore re-evaluated their archived data using varying thresholds. This analysis confirmed an attenuation of RNA expression difference between frontal and temporal cortex in autistic brain compared to control brain when no LFC threshold is used. However, the attenuation of differential RNA expression between control and autistic brain is not noted when a LFC threshold ≥1 is used (Supplementary Table 2). Thus, the cortical gene expression differences reported by Voineagu et al. are not large in magnitude. Unlike in our dataset, however, there was not a high correlation between the fold change values in the autistic and control groups, suggesting that greater variability in the autistic group was not likely to be solely responsible for the difference between the two groups in the study by Voineagu et al. (Supplementary Fig. 2).
Although Voineagu et al. also studied gene expression profiles of cerebellar hemisphere4, they did not evaluate their data regarding differential gene expression of cerebellum vs. either frontal or temporal cortex in control brain compared to autistic brain. We therefore analyzed their archived data from these three brain regions with respect to this issue. We found that the results of comparisons of gene expression between cerebellum and frontal cortex or between cerebellum and temporal cortex were similar to each other and similar to our data comparing cerebellar cortex and occipital cortex (Supplementary Tables 3, 4; Supplementary Fig. 3).
Discussion
Because of the prevalence of autism and its personal and societal impacts, the determination of its etiologies and pathogenetic processes and the development of effective therapies are public health priorities. At this time, most cases are idiopathic although many rare and uncommon causes of autism are now recognized. Numerous pathogenetic processes for autism have been proposed. Neurophysiological and, especially, functional neuroimaging data reveal abnormal functional connectivity in autistic brains6,7. This is likely consequent to multiple underlying primary conditions and has lead to differing models of an altered brain connectome in autism8,9. Voineagu et al. recently observed that there is an attenuation of regional gene expression differentiation in autistic brain between frontal and temporal cortex4. That work provides important biochemical data in support of the general hypothesis of functional regional disconnectivity in autistic brain.
We sought to determine if other areas of autistic brain show a similar attenuation of differences of gene expression. To date, there are few whole genome transcriptomic analyses of autistic brain and still fewer that include both transcriptomic analyses of multiple regions of brain and an adequate number of independent brain specimens to allow for comprehensive statistical evaluation. Our work indicates that the attenuation of gene expression differences that was found in autistic frontal vs. temporal cortex is not generalizable to all cortical regions as it is not noted in occipital cortex vs. cerebellar cortex. Furthermore, we analyzed archived whole genome transcriptomic data to compare differences between frontal cortex and cerebellum and between temporal cortex and cerebellum in autistic vs. control brain4. The results of these analyses indicate a lack of attenuation of gene expression differences between these regions in autistic brain and are similar to the analysis of our data regarding cerebellar cortex and occipital cortex.
Our findings, and the results of our analyses of the cerebellar gene expression data of Voineagu et al., contrast with the conclusions of Ziats and Rennert who analyzed differentially expressed RNAs between prefrontal cortex and cerebellum using two autistic and two control brains. They reported reduced regional transcriptional differentiation in the autistic brain samples, both for messenger RNA and non-coding RNA10. However, the small number of brains studied by Ziats and Rennert prevent assessment of the effect of RNA expression variability whose importance is highlighted in this study.
Overall, our data do not indicate significant differences in cerebral cortical-cerebellar cortical gene expression differentiation between autistic and control brains. This is in contrast to the differences between frontal and temporal cortical differentiation noted earlier. Taken together, our data and that of Voineagu et al. suggest that abnormal regional gene expression differentiation may be limited to specific cerebral cortical regions, and that such perturbations are significant but subtle at a transcriptomic level. Our findings also indicate that studies of regional differential gene expression need to address variability within groups.
Methods
Subjects and samples
Brain tissue samples from Brodmann area 19 (BA19) occipital cortex and cerebellar hemispheric cortex were procured from subjects and controls through the Autism Tissue Program (ATP, www.atpportal.org) from the Harvard Brain Tissue Resource Center (www.brainbank.mclean.org) and the National Institute for Child Health and Human Development (NICHD) Brain and Tissue Bank (www.btbank.org). Tissues were procured from a total of 9 autism and 9 control subjects. Inclusion and exclusion criteria and details regarding each subject and brain specimen are noted elsewhere5. This study was approved by the Institutional Review Board of the Cleveland Clinic and is in accord with the principles of the Declaration of Helsinki. The gene expression data presented in this publication have been deposited in NCBI Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series accession number GSE38322.
Sample processing
Total DNA and RNA were co-extracted from ~40 mg of cerebellar cortex and ~40 mg BA19 cortex and RNA and DNA were tested for purity and concentration as described5. Samples of RNA were processed and applied to Illumina HumanRefHT12 v4 BeadChip gene expression microarrays according to manufacturer's instructions.
Statistical analyses
Data were imported from Genome Studio (Illumina) to the R statistical environment (http://www.r-project.org/) and processed via standard Bioconductor packages: lumi, made4, and limma (www.Bioconductor.org/packages/release/bioc/). Expression data from the full dataset were quantile-normalized, variance-stabilized, and filtered from probes with ≤ 0.01 detection p-values. Differential expression analyses were then performed separately for autism and control tissue in our own dataset and that of Voineagu and others4 (GEO accession: GSE30573), who utilized the SAM package for their statistical analyses. Differentially expressed probes (between regions, within the autism or control group) were determined using a linear modeling approach in limma, with results filtered by varying thresholds for p-value and log fold change. The relationship between differential regional gene expression in autistic and control brain tissue was also explored with simple linear regression. Coefficient of determination was used to assess correlations between autistic and control groups for log fold changes or p-values of probe-wise comparisons between regions.
Bioinformatics analysis
For gene ontology analysis of differentially expressed genes, we submitted differentially expressed probes to the DAVID bioinformatics tool (http://david.abcc.ncifcrf.gov/)11. The list was compared with a background of the total set of expressed genes in our samples (detection p-value ≤ 0.01) and functional annotation clustering was performed. Default settings for medium stringency (in DAVID version 6.7) were used and the top 6 functional annotation clusters were reported with enrichment scores11,12. Enrichment scores are the negative log10 of p-values based on Fisher's exact test. Ontology terms related to cellular localization, gene product function, and biological pathway were included in the analysis, and clusters were manually annotated with representative terms for the set of annotation terms in a cluster.
Author Contributions
M.R.G. and M.R.N. designed the study. M.R.G. and R.A.R. performed the statistical analyses. M.R.N. supervised the study and wrote the initial draft of the manuscript. All authors read and revised the manuscript before the submission.
Supplementary Material
SUPPLEMENTARY MATERIALS
Acknowledgments
We are grateful to the families who provided specimens from their loved ones for research and for the efforts of the Autism Tissue Program, Harvard Brain Tissue Resource Center and the National Institute for Child Health and Human Development Brain and Tissue Bank. We thank the Autism Research Institute and the Research Program Committee of the Cleveland Clinic for support of this work.
References
- Levy S. E., Mandell D. S. & Schultz R. T. Autism. Lancet 374, 1627–1638 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur C. Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res. 1380, 42–77 (2011). [DOI] [PubMed] [Google Scholar]
- Amaral D. G. The promise and pitfalls of autism research: an introductory note for new autism researchers. Brain Res. 1380, 3–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineagu I. et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474, 380–384 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M. R., Rubin R. A., Falcone T., Ting A. H. & Natowicz M. R. Brain transcriptional and epigenetic associations with autism. PLoS One 7, e44736 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew N. J. & Keller T. A. The nature of brain dysfunction is autism: functional brain imaging studies. Curr. Opin. Neurol. 23, 124–130 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameis S. H. & Szatmari P. Imaging-genetics in autism spectrum disorder: advances, translational impact, and future directions. Front. Psychiatr. 3, e46 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller R.-A. et al. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb. Cortex 21, 2233–2243 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter G. S. Functional magnetic resonance imaging of autism spectrum disorders. Dialogues Clin. Neurosci. 14, 319–351 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziats M. N. & Rennert O. M. Aberrant expression of long noncoding RNAs in autistic brain. J. Mol. Neurosci. 29, 589–593 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D. W., Sherman B. T. & Lempicki R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protoc. 4, 44–57 (2009). [DOI] [PubMed] [Google Scholar]
- Hosack D. A., Dennis Jr G., Sherman B. T., Lane H. C. & Lempicki R. A. Identifying biological themes within lists of genes with EASE. Genome Biol. 4, R70 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY MATERIALS


