Abstract
Insulin-like growth factor I (IGF-I) is implicated in breast cancer development and 1, 25-dihydroxyvitamin D3 (1, 25-D3) has been shown to attenuate prosurvival effects of IGF-I on breast cancer cells. In this study the role of IGF binding protein-3 (IGFBP-3) in 1, 25-D3-induced apoptosis was investigated using parental MCF-7 breast cancer cells and MCF-7/VDR cells, which are resistant to the growth inhibitory effects of 1, 25-D3. Treatment with 1, 25-D3 increased IGFBP-3 mRNA expression in both cell lines but increases in intracellular IGFBP-3 protein and its secretion were observed only in MCF-7. 1, 25-D3-induced apoptosis was not associated with activation of any caspase but PARP-1 cleavage was detected in parental cells. IGFBP-3 treatment alone produced cleavage of caspases 7, 8, and 9 and PARP-1 in MCF-7 cells. IGFBP-3 failed to activate caspases in MCF-7/VDR cells; however PARP-1 cleavage was detected. 1, 25-D3 treatment inhibited IGF-I/Akt survival signalling in MCF-7 but not in MCF-7/VDR cells. In contrast, IGFBP-3 treatment was effective in inhibiting IGF-I/Akt pathways in both breast cancer lines. These results suggest a role for IGFBP-3 in 1, 25-D3 apoptotic signalling and that impaired secretion of IGFBP-3 may be involved in acquired resistance to vitamin D in breast cancer.
1. Introduction
The insulin-like growth factor I (IGF-I) system is essential for normal growth and development. IGF-I is known to modulate control by insulin of normal carbohydrate and lipid metabolism. In addition, IGF-I has been reported to play a role in several pathological conditions. Interaction with the IGF binding proteins (IGFBPs) has been shown to both enhance and attenuate actions of IGF-I [1]. In addition, the IGFBPs are known to possess intrinsic growth regulatory activity, independent of their interactions with IGF-I. Insulin-like growth factor I (IGF-I) is implicated in breast cancer development and has been shown to rescue breast cancer cells from apoptosis induced by a range of chemotherapeutic agents [2]. Cellular responsiveness to IGF-I growth stimulation depends on the expression and activity of the signal transducing IGF-I receptor (IGF-IR) and a family of structurally related insulin-like growth factor binding proteins (IGFBP-1 to IGFBP-7). The major carrier of IGF-I in the circulation is IGFBP-3, which has been shown to inhibit cell growth and induce apoptosis in several cancer cell lines [3]. IGFBP-3 has been shown to regulate cell growth through both IGF-IR-dependent and -independent mechanisms (reviewed in [4]). The latter may involve signalling through an alternative cell surface receptor [5] or may involve direct nuclear actions by IGFBP-3 [6].
A number of factors with potent growth-inhibitory and apoptosis-inducing effects have been shown to induce the expression and secretion of IGFBP-3 in breast cancer cell lines, including 1, 25-dihydroxyvitamin D3 (1, 25-D3), the active metabolite of vitamin D3 which has been shown to inhibit breast cancer cell growth [7]. This finding suggests that IGFBP-3 may mediate or facilitate the inhibitory effects of 1, 25-D3. The aim of our study was to evaluate the role of IGFBP-3 in 1, 25-D3-induced apoptosis in breast cancer cells. To this end, IGFBP-3 expression and secretion were investigated in parental MCF-7 breast cancer cells and the 1, 25-D3-resistant cell line MCF-7/VDR. This cell line is a vitamin-D-resistant clone of MCF-7 cells, which was developed by incubation of parental cells with a low concentration of 1, 25-D3, separating out the viable (resistant) cells and repeating this procedure with increasing concentrations of 1, 25-D3 [8]. This cell line contains fully functional VDR, although in a lower number than seen with the parental MCF-7 cells. The regulation of the 24-hydroxylase enzyme appeared to be intact and no differences with regard to growth rate and morphological appearance between parental and resistant clone were observed. The MCF-7/VDR cell line thus provides a valuable tool for identifying the exact mechanism of action of vitamin D and the development of vitamin D resistance.
2. Materials and Methods
2.1. Cell Culture and Reagent
MCF-7 human breast cancer cells were obtained from the European tissue culture collection and used between passages 5 and 20. Vitamin-D-resistant MCF-7/VDR cells were obtained as a gift from Dr. Mork Hansen [8]. Both parental and resistant cells were grown in RPMI 1640 supplemented with 2 mM of glutamine, 100 IU/mL of penicillin, 100 μg/mL of streptomycin, and 2% of foetal bovine serum (FBS). 1, 25-D3 (Sigma UK) was used at a concentration of 100 nM and IGFBP-3 (R&D Systems) up to 100 nM.
2.2. Viability Assay
MCF-7 and MCF-7/VDR cells were seeded into 24 well plates at a density of 1 × 104 cells/well. After 24 h, cells were treated with reagents or vehicle for up to six days. At the end of the incubation period, medium was removed and cells were incubated with neutral red solution (40 μg/mL in phenol red-free medium) for 2 h at 37°C. Following washing, fixation, and solubilisation, absorbance at 550 nm was determined.
2.3. Western Blot Analysis
Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer containing 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 1X PBS. Equal amounts of protein (30 μg per lane) were subjected to SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked with 5% milk in 0.05% Tween-20/TBS and then incubated with the primary antibody of interest overnight. Membranes were then incubated with the appropriate secondary horseradish-peroxidase-conjugated antibody. Bands were visualised using the enhanced chemiluminescence Western blotting detection system (ECL, Amersham). Anticleaved caspases 7, 8, and 9 and Poly [ADP-ribose] polymerase 1 (PARP-1) and antitotal caspases 7, 8, and 9, phospho-Akt, and PARP-1 antibodies were purchased from Cell Signalling. Anti-β-actin (Sigma Aldrich) was used as a loading control.
2.4. RNA Isolation and cDNA Synthesis
Total RNA from cells was extracted by using the PureLink RNA Mini-kit (Invitrogen). The quantity and the quality of RNA extracted was estimated by Nano-drop Spectrophotometer. For the reverse transcription, 2 μg of RNA was resuspended in 10 μL of nuclease free water with 2 μL random hexamer (50 μg) and was incubated at 70°C for 5 min. Then, the samples were resuspended with 13 μL of Master Mix (5 μL RT 5X Buffer, 2.5 μL of dNTP 10 mM, 0.5 μL Rnase OUT 40 U/μL, 0.5 μL of Reverse Transcriptase (MMLV, Promega), and 3.5 μL of Nuclease Free Water). This mix was run for 1 h at 42°C, 5 min at 95°C, and 5 min at 4°C. The cDNA was stored at −20°C.
2.5. RT-PCR Analysis of IGFBP-3 mRNA
The primers used to amplify IGFBP-3 and 28S rRNA were IGFBP-3 forward (GAAGGGCGACACTGCTTTTTC), IGFBP-3 reverse (CCAGCTCCAGGAAATGCTAG), 28S forward (GTTCACCCACTAATAGGGAAC), and 28S reverse (GGATTCTGACTTAGAGGCGTT). PCR was carried out in a total volume of 50 μL containing 3 μL of cDNA sample and 10 μM sense and antisense primers. The RT-PCR exponential phase was determined in 28 to 33 cycles to allow quantitative comparisons. IGFBP-3 cDNA was amplified at 94°C for 2 minutes followed by 33 cycles at 94°C for 45 seconds, 63°C for 45 sec, and 72°C for 1 minute. 28S cDNA was amplified at 94°C for 2 minutes followed by 28 cycles at 94°C for 45 seconds, 58°C for 45 sec, and 72°C for 1 minute. Final extension was performed at 72°C for 5 min. Amplification products (8 μL) were resolved in 2% agarose gel, stained with ethidium bromide, and visualized under UV light.
2.6. Detection of IGFBP-3 Secretion in Medium by ELISA Assay
IGFBP-3 protein level in each 200 μL of medium and 100 μg of cell extract was determined using a human IGFBP-3 ELISA kit (RayBioTech, USA) according to the manufacturer's protocol.
2.7. Antibody Specific Array
Mitogen-activated protein kinases (MAPK) protein phosphorylation was measured in each 300 μg of cell extracts using Human Phospho-MAPK Array Kit according to the manufacturer (Proteome Profiler; R&D Systems). Briefly, antibody array membranes were incubated with protein lysates and then incubated with antibody array biotinylated antibody. Finally the membranes were incubated with streptavidin HRP-conjugated antibody. Immunoreactivity was visualized using a chemiluminescent substrate. Densitometric analysis was performed using GS-800 Calibrated Densitometer (Bio-Rad, UK).
2.8. Statistics
Data are reported as mean ± SD and analyzed with one-way ANOVA followed by the Bonferroni posttest for multiple comparisons using GraphPad Prism version 4.0. A value of P < 0.05 was considered significant.
3. Results
3.1. Effects of 1, 25-D3 on Growth and IGFBP-3 Expression in Parental MCF-7 and Resistant MCF-7/VDR Cells
MCF-7 and MCF-7/VDR cells were treated with increasing concentrations of 1, 25-D3 for up to 6 days. Cell viability was examined by neutral red dye assay (Figure 1(a)). Whilst 1, 25-D3 significantly decreased viability of MCF-7 cells, it had no significant effect on MCF-7/VDR cell viability (Figure 1(a)). To determine effects on IGFBP-3 mRNA expression, MCF-7 and MCF-7/VDR cells were treated with 100 nM 1, 25-D3 for up to 5 days. Whole RNA was extracted from the cells at different times of treatment and IGFBP-3 expression was examined by RT-PCR (Figure 1(b)). In both MCF-7 and MCF-7/VDR cells, 1, 25-D3 treatment enhanced IGFBP-3 mRNA expression indicating that 1, 25-D3 was effective in inducing IGFBP-3 mRNA expression irrespective of the observed resistance in MCF-7/VDR cells to 1, 25-D3-induced apoptosis (Figure 1(b)).
Figure 1.
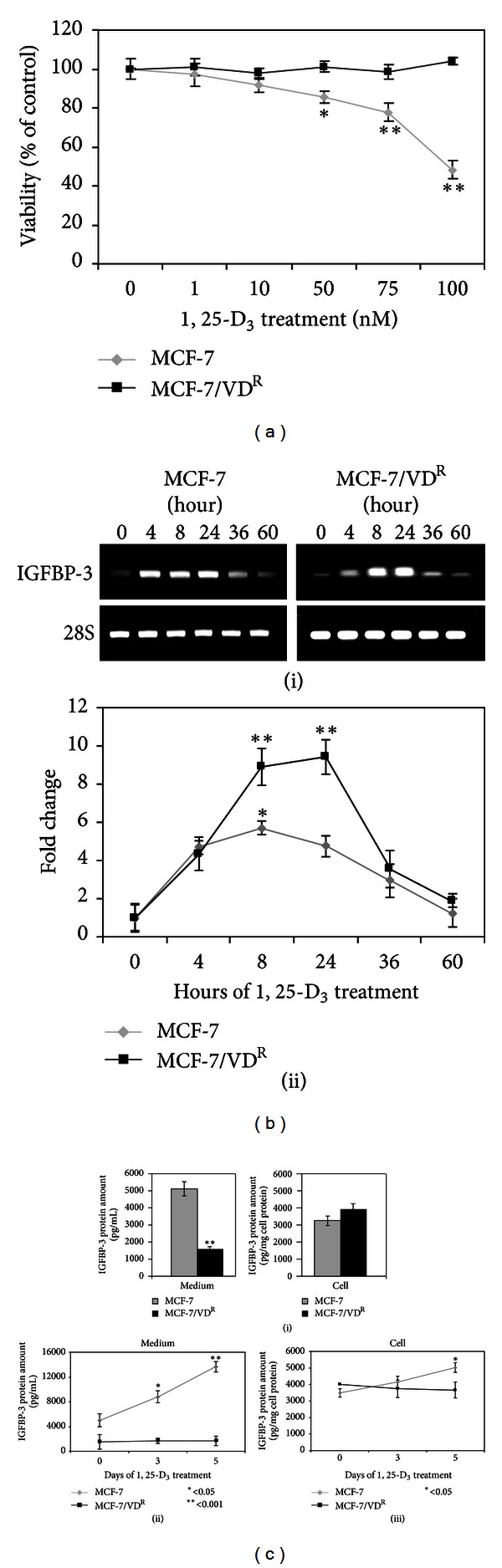
Effect of 1, 25-D3 on MCF-7 and MCF-7/VDR cell viability and IGFBP-3 expression. (a) MCF-7 and MCF-7/VDR cells were treated with increasing concentrations of 1, 25-D3 (up to 100 nM) or 0.1% ethanol vehicle as a control for 6 days. Cell viability was determined by neutral red assay. Means of 3 separate experiments are shown. *P < 0.05 and **P < 0.001 are statistically significant compared to the control. (b) (i) MCF-7 and MCF-7/VDR cells were treated with 100 nM 1, 25-D3 for up to 60 hours. IGFBP-3 mRNA expression was measured by RT-PCR. 28S mRNA expression was used as house-keeping gene. Nontreated cells were used as controls. (ii) Densitometric analysis of of IGFBP-3 mRNA expression. Data shown means of three separate experiments. (c) Intracellular IGFBP-3 levels and secretion into medium was determined by ELISA in MCF-7 and MCF-7/VDR cells. (i) IGFBP-3 expression and secretion into the medium in untreated MCF-7 and MCF-7/VDR cells. (ii) IGFBP-3 secretion into the medium in MCF-7 and MCF-7/VDR cells treated with 100 nM of 1, 25-D3 for up to 5 days quantitated by ELISA. (iii) The amount of intracellular IGFBP-3 production by MCF-7 and MCF-7/VDR cells treated with 100 nM of 1, 25-D3 was measured at day 0, 3, and 5 by ELISA. Means of 3 separate experiments are shown. *P < 0.05 and **P < 0.001 are statistically significant compared to the control.
Next, we examined effects of 1, 25-D3 on IGFBP-3 at the level of protein expression and secretion in MCF-7 versus MCF-7/VDR cells. Cells were treated with 100 nM 1, 25-D3 for up to 5 days. The amount of IGFBP-3 protein present in the cell or secreted into the medium was assessed by ELISA. The basal level of intracellular IGFBP-3 protein expression was found to be similar in both cell lines (P > 0.05); however, the amount of IGFBP-3 protein in medium conditioned by parental MCF-7 cells was significantly higher than for the resistant cell line (P < 0.001), indicating a reduced secretion of IGFBP-3 by the MCF-7/VDR cells (Figure 1(c)). In addition, 1, 25-D3 treatment induced IGFBP-3 protein expression and secretion in MCF-7 but not in MCF-7/VDR cells (P < 0.05 and P < 0.001, resp.). Taken together, these results showed that impaired secretion but not transcriptional regulation of IGFBP-3 is associated with resistance of MCF-7/VDR to 1, 25-D3.
3.2. 1, 25-D3- and IGFBP-3-Induced Apoptosis in MCF-7 and MCF-7/VDR Cells
To compare characteristics of 1, 25-D3- and IGFBP-3-induced apoptosis, parental and MCF-7/VDR cells were treated for 5 days with 100 nM 1, 25-D3 or 100 nM IGFBP-3. Activation of caspases 7, 8, and 9 was monitored by detection of cleaved (active) caspase fragments by immunoblotting. In addition, PARP-1 cleavage was examined and β-actin was used as a house-keeping protein (Figure 2). 1, 25-D3 treatment did not lead to activation of any caspase but induced PARP-1 cleavage in parental MCF-7 but not in MCF-7/VDR cells. In contrast, IGFBP-3 treatment produced cleavage of caspases 7, 8, and 9 and PARP-1 in MCF-7 cells. IGFPB-3 failed to activate caspases in MCF-7/VDR cells; however PARP-1 cleavage was detected indicating an alternative pathway by which the protein induces apoptosis in these vitamin-D-resistant cells.
Figure 2.
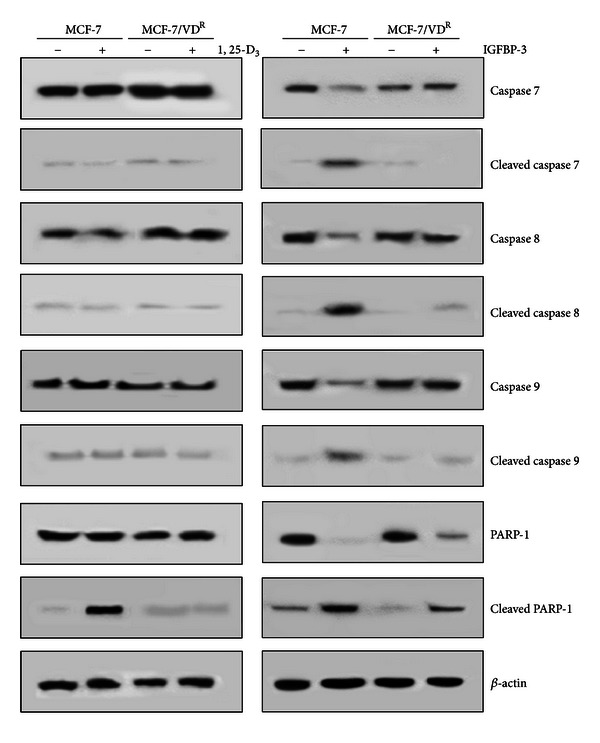
Caspase activation in response to 1, 25-D3 and IGFBP-3. MCF-7 and MCF-7/VDR cells were treated with 100 nM 1, 25-D3 or 0.1% ethanol as vehicle control for 5 days (left hand panel) or 100 nM IGFBP-3 for 3 days. Control cells received an equal volume of PBS diluent (right-hand panel). Whole cell extracts were prepared and analysed by immunoblotting using specific antibodies of interest and β-actin was used as a loading control. Data shown are representative of three identical experiments.
3.3. Effect of 1, 25-D3 and IGFBP-3 on IGF-I/Akt Survival Signalling in MCF-7 and MCF-7/VDR Cells
While parental MCF-7 cells do not express detectable IGF-I [9], the cells respond to the mitogenic and antiapoptotic effects of exogenous IGF-I and previous experiments have demonstrated that vitamin D treatment can attenuate the survival effect of IGF-I in parental MCF-7 cells [10]. To compare effects on IGF-I-mediated cell survival, MCF-7 and MCF-7/VDR cells were treated with 100 nM 1, 25-D3 and 30 nM IGF-I, alone or in combination in serum-free medium and cell viability was examined by neutral red dye assay. Cells were also cultured in medium supplemented with 2% foetal bovine serum as a control. For both cell lines serum deprivation induced up to 70–80% of cell death compared to cells cultured in the presence of serum (P < 0.001) and addition of IGF-I to serum-free medium rescued cell viability (P > 0.05 compared to control). 1, 25-D3 treatment attenuated prosurvival effects of IGF-I in parental but not in resistant MCF-7/VDR cells (Figure 3(a)). Failure of 1, 25-D3 to modulate IGF-I survival signalling in resistant cells could be due to differential regulation of IGF-I bioavailability by IGFBPs such as IGFBP-3, which is not secreted by these cells.
Figure 3.
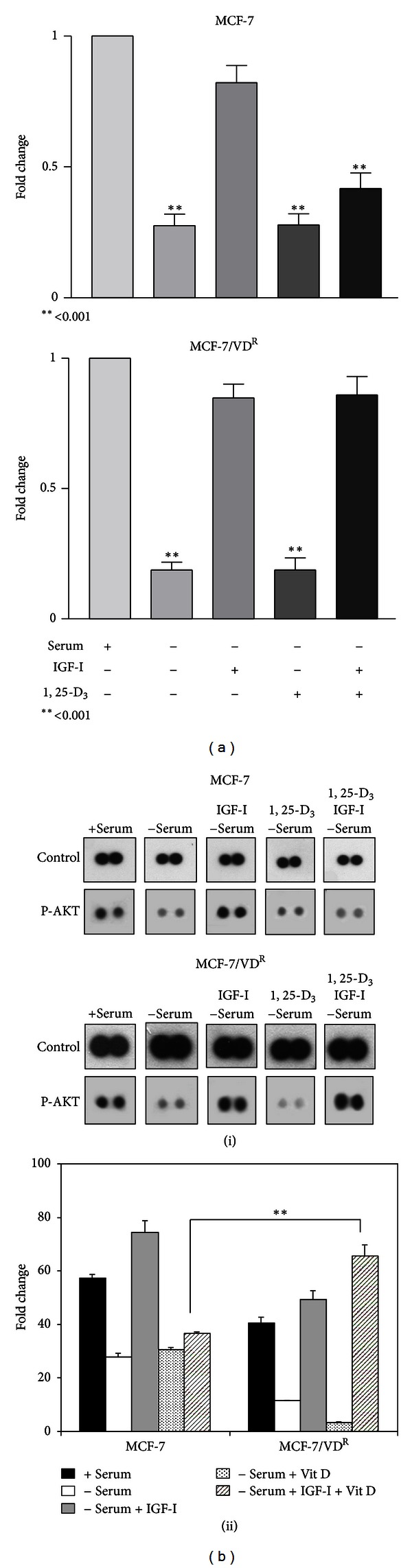
Modulation of IGF-induced Akt phosphorylation in response to 1, 25-D3 treatment in MCF-7 and MCF-7/VDR cells. (a) MCF-7 and MCF-7/VDR cells were treated with 100 nM 1, 25-D3 or 30 nM IGF-I, alone or in combination in serum-free medium. Cells were also cultured in medium supplemented with 2% serum as a control. After 6 days of treatment, cell viability was estimated by neutral red assay. *P < 0.05 and **P < 0.001 are statistically significant compared to control. Means of 3 separated experiments are shown (n = 12). (b) (i) MCF-7 and MCF-7/VDR cells were treated with 100 nM 1, 25-D3 or 30 nM IGF-I, alone or in combination, in serum-free medium. After 5 days of treatment, whole cell extracts were prepared and analysed on a phospho-MAPK antibody array (R&D Systems, UK) following manufacturer's instruction. (ii) Densitometric analysis of Akt phosphorylation. Data shown are means of 3 replicates, significantly different from parental cells. **P < 0.001.
We next compared 1, 25-D3 and IGFBP-3 treatment on MAPK and Akt activation in parental and resistant cells since it is well documented that IGF-I/MAPK and IGF-I/Akt signalling plays a crucial role in proliferation and survival of breast cancer cells. Cells were treated for 5 days with 100 nM 1, 25-D3 and 30 nM IGF-I, alone or in combination in serum-free medium. Cells were collected and isolated proteins were analysed on human phospho-MAPK antibody array. With respect to Akt phosphorylation, 1, 25-D3 attenuated the positive effect of IGF-I on activation of Akt in MCF-7 cells but failed to do so in MCF-7/VDR cells (Figure 3(b)). No significant differences in activation of ERK, JNK, and p38 were detected between the two cell lines with these treatments (data not shown). Differential effects of 1, 25-D3 in parental and resistant cells on IGF-I-stimulated Akt activation were confirmed by immunoblotting. In contrast, treatment with IGFBP-3 reduced IGF-I-stimulated Akt phosphorylation in both cell lines (Figures 4(a) and 4(b)).
Figure 4.
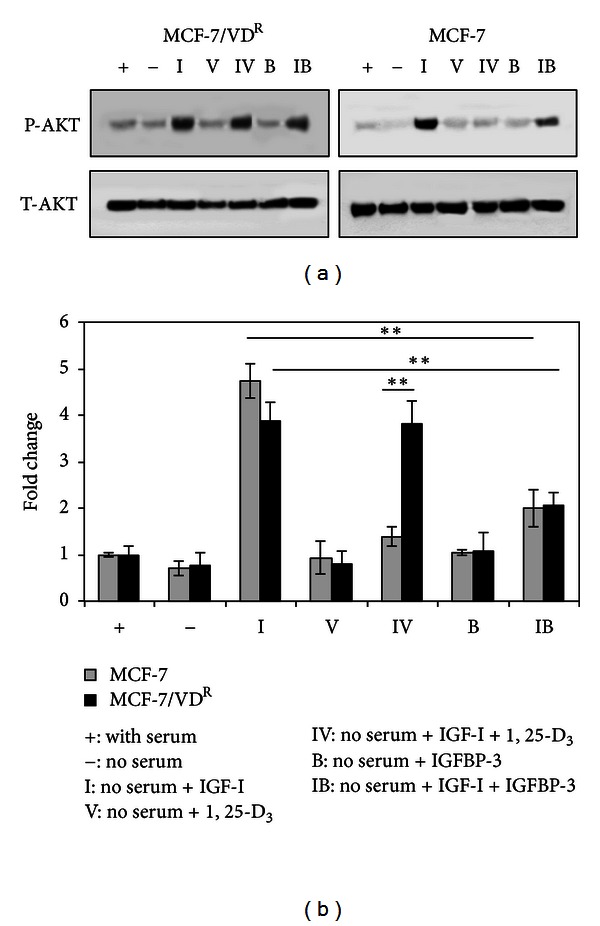
Differential modulation of IGF-induced Akt phosphorylation in response to 1, 25-D3 and IGFBP-3 treatment in MCF-7 and MCF-7/VDR cells. (a) MCF-7 and MCF-7/VDR cells were treated with 100 nM 1, 25-D3 or 30 nM IGF-I, alone or in combination, in serum-free medium. Cells were also treated with 100 nM IGFBP-3 alone or in combination with 30 nM IGF-I in serum-free medium. After 5 days of treatment, whole cell extracts were prepared and analysed by immunoblotting for total-Akt (T-Akt) and phospho-Akt (P-Akt). (b) Densitometric analysis of immunoblots was performed using GS-800 Calibrated Densitometer (Bio-Rad UK). Data shown are representative of three identical experiments. Means of 3 separated experiments are shown. *P < 0.05 and **P < 0.001 are statistically significant.
4. Discussion
The IGFBPs are secreted proteins, which bind to IGFs with high affinity. The IGFBP family has 7 distinct subgroups, IGFBP-1 through 7, and their production is tissue-type specific. Approximately 98% of IGF-1 is always bound to one of these binding proteins. The IGFBPs help to lengthen the halflife of circulating IGFs in all tissues and enhance or attenuate IGF signaling depending on their physiological context. IGFBP-3 is the most abundant of the family and accounts for 80% of all IGF binding [11]. IGFBP-3 is known to control IGF-I signalling leading to differential regulation of cell growth and apoptosis [12, 13]. A number of growth factors and hormones, including 1, 25-D3, have been shown to induce the expression of IGFBP-3 in breast cancer cell lines [7]. Comparative expression profiling of human IGFBP genes in different cancer cells demonstrated that IGFBP-1, -3 and -5 are primary 1, 25-D3 target genes [14]. In breast cancer, it was shown that 1, 25-D3 causes cyclical IGFBP-3 mRNA accumulation with a periodicity of 60 min [15]. Accordingly, VDR also showed cyclical ligand-dependent association with the chromatin regions of its VDREs. Interestingly, HDAC4 and HDAC6 proteins, which are upregulated in a cyclical fashion in response to 1, 25-D3, show cyclical VDR ligand-induced association with VDRE regions of the IGFBP-3 gene. Available evidence indicates that IGFBP-4 and 6 are not primary 1, 25-D3 target genes [14]. IGFBP-5 can colocalize with VDR in the nucleus and modulate vitamin D responses in osteoblasts [16]. IGFBP-6 has also been reported to interact with VDR in the bone [17].
Although there is an increasing body of evidence that 1, 25-D3 exerts potent regulatory effects on breast cancer cell growth, differentiation, and apoptosis [18], the mechanisms involved are not fully understood. Our results demonstrate that 1, 25-D3 treatment leads to an increase in IGFBP-3 mRNA in both sensitive and resistant MCF-7 cell lines, suggesting that the resistance to 1, 25-D3 is not due to impairment in IGFBP-3 gene expression at the mRNA level. This result was not surprising because the MCF-7/VDR cells have been reported to express a functional Vitamin D receptor [8]. We next determined if there was a difference between sensitive and resistant cells at the level of IGFBP-3 protein. Whilst there was no difference in basal intracellular IGFBP-3 protein expression, the level of IGFBP-3 in conditioned medium from resistant cells was significantly lower than medium from parental MCF-7 cells. Furthermore, we detected a clear impairment of increased expression and secretion of this protein in MCF-7/VDR cells in response to 1, 25-D3 treatment compared to parental cells, suggesting that effective secretion of this protein facilitates 1, 25-D3 responsiveness. A functional role of secreted versus nonsecreted IGFBP-3 is an interesting issue in the literature. One study demonstrated that nuclear translocation of IGFBP-3 and induction of apoptosis in parental MCF-7 cells require IGFBP-3 secretion and reuptake [19]. In contrast, Battacharyya and colleagues [20] reported that secreted and non-secreted IGFBP-3 may be functionally equivalent in induction of apoptosis in prostate cancer cells. Several studies have indicated that structural modifications such as glycosylation [21] and phosphorylation [22] can affect IGFBP-3 binding activity. However an ELISA approach, as used in our study, was unable to detect any such differences in secreted IGFBP-3 from MCF-7 cells.
It has been previously reported that 1, 25-D3 and IGFBP-3 induce MCF-7 cell death [23, 24]. Available evidence suggests differences in the characteristics of 1, 25-D3- and IGFBP-3-induced apoptosis in MCF-7 cells. It was previously reported that 1, 25-D3 induced apoptosis in a caspase-independent manner in MCF-7 cells [25] and this is confirmed in our present study. Whilst we found that 1, 25-D3 treatment did not induce activation of caspases 7, 8, and 9 in either MCF-7 or MCF-7/VDR cell line, PARP-1 cleavage was detected in parental but not resistant cells. Indeed, it has previously been demonstrated that PARP-1 cleavage associated with 1, 25-D3-induced apoptosis could involve other proteinases such as calpains [26]. In contrast, we found that exogenous IGFBP-3-stimulated activation of caspases 7, 8 and 9 in parental MCF-7 but not in MCF-7/VDR cells. However PARP-1 cleavage was detected in response to IGFBP-3 treatment in both cell lines suggesting that IGFBP-3 produces PARP-1 cleavage in a caspase-independent manner in MCF-7/VDR cells. PARP-1 processing leading to activation of nucleases and DNA fragmentation appears as a key point in the execution phase of apoptosis. In support of our findings other reports have demonstrated PARP-1 processing in the absence of any caspase activation suggesting the role of other proteases in this process [27–29]. The ability of IGFBP-3 to induce apoptosis by both caspase-dependent and caspase-independent mechanisms suggests that this protein could act through two different signalling pathways. This observation also suggests that biochemical properties of endogenous and secreted IGFBP-3 may differ from exogenous protein.
A number of studies have indicated IGF-I-dependent and -independent mechanisms by which IGFBP-3 induces apoptosis. By limiting IGF-I bioavailability, IGFBP-3 controls signal transduction through the IGF-I receptor, including survival signalling and induction of cell death. Exogenous IGFBP-3 also appears to exert IGF-I-independent effects, activating apoptosis via novel or death receptor pathways [9, 30, 31]. In contrast, other studies have shown that IGFBP-3 modulates RXR/Nur77 signalling in the nucleus, thereby inducing apoptosis in a mitochondria-dependent manner [32]. We found that RXR-α is expressed only in parental MCF-7 but not in MCF-7/VDR cells (unpublished observations) suggesting that a RXR/Nur pathway does not exist in these cells and this is supported by absence of caspase 9 activation in response to IGFBP-3.
It is well documented that Akt activation plays a crucial role in antiapoptotic actions of IGF-I in breast cancer cells and our initial experiments clearly demonstrated that 1, 25-D3 treatment attenuated the survival effect of IGF-I in parental cell line but not in resistant MCF-7/VDR cells. Our results using MAPK/Akt antibody array analysis demonstrated that 1, 25-D3 attenuated IGF-I-induced Akt phosphorylation in MCF-7 cells but failed to do so in MCF-7/VDR cells, suggesting that failure to modulate IGF-I/Akt survival signalling could contribute to the resistance of this cell line to 1, 25-D3. In contrast, induction of apoptosis by exogenous IGFBP-3 in both 1, 25-D3-sensitive and -resistant cells was associated with inhibition the IGF-I/Akt pathway (Figure 5). In this regard the ability of IGFBP-3 to downregulate Akt activity in Her2 overexpressing MCF-7 cells has been previously reported [33].
Figure 5.
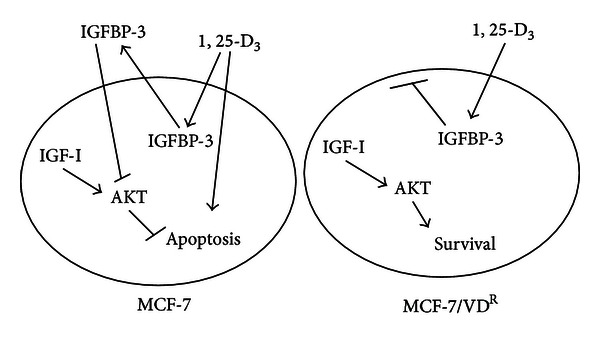
Proposed interaction between 1, 25-D3 and IGFBP-3 in MCF-7 and MCF-7/VDR cells. In parental cells stimulation by 1, 25-D3 of IGFBP-3 secretion attenuates IGF-1-induced activation of Akt, leading to apoptosis. In addition, 1, 25-D3 may initiate caspase-independent pathways contributing to cell death in parental cells. In resistant cells, failure of IGFBP-3 secretion is associated with activation of the IGF-I/Akt pathway, leading to cell survival.
5. Conclusion
Taken together our results suggest a role for IGFBP-3 in 1, 25-D3 apoptotic signalling and impaired secretion of IGFBP-3 may be involved in acquired resistance to vitamin D in breast cancer cells. In addition, regulation of IGF-I/Akt survival signalling may function as a key point of convergence that may determine breast cancer cell fate in response to 1, 25-D3/IGFBP-3.
Acknowledgment
A grant from the European Union (Marie Curie RTN NucSys) supported this research.
References
- 1.Martin JL, Baxter RC. Signalling pathways of insulin-like growth factors (IGFs) and IGF-binding protein -3. Growth Factors. 2011;29(6):235–240. doi: 10.3109/08977194.2011.614237. [DOI] [PubMed] [Google Scholar]
- 2.Le Bourhis X, Toillon RA, Boilly B, Hondermarck H. Autocrine and paracrine growth inhibitors of breast cancer cells. Breast Cancer Research and Treatment. 2000;60(3):251–258. doi: 10.1023/a:1006461621905. [DOI] [PubMed] [Google Scholar]
- 3.Martin JL, Coverley JA, Pattison ST, Baxter RC. Insulin-like growth factor-binding protein-3 production by MCF-7 breast cancer cells: Stimulation by retinoic acid and cyclic adenosine monophosphate and differential effects of estradiol. Endocrinology. 1995;136(3):1219–1226. doi: 10.1210/endo.136.3.7532580. [DOI] [PubMed] [Google Scholar]
- 4.Jogie-Brahim S, Feldman D, Oh Y. Unraveling insulin-like growth factor binding protein-3 actions in human disease. Endocrine Reviews. 2009;30(5):417–437. doi: 10.1210/er.2008-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh Y, Muller HL, Lamson G, Rosenfeld RG. Insulin-like growth factor (IGF)-independent action of IGF-binding protein-3 in Hs578T human breast cancer cells. Cell surface binding and growth inhibition. Journal of Biological Chemistry. 1993;268(20):14964–14971. [PubMed] [Google Scholar]
- 6.Liu B, Lee HY, Weinzimer SA, et al. Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid X receptor-α regulate transcriptional signaling and apoptosis. Journal of Biological Chemistry. 2000;275(43):33607–33613. doi: 10.1074/jbc.M002547200. [DOI] [PubMed] [Google Scholar]
- 7.Colston KW, Perks CM, Xie SP, Holly JMP. Growth inhibition of both MCF-7 and Hs578T human breast cancer cell lines by vitamin D analogues is associated with increased expression of insulin-like growth factor binding protein-3. Journal of Molecular Endocrinology. 1998;20(1):157–162. doi: 10.1677/jme.0.0200157. [DOI] [PubMed] [Google Scholar]
- 8.Hansen CM, Rohde L, Madsen MW, et al. MCF-7/VDR: a new vitamin D resistant cell line. Journal of Cellular Biochemistry. 2001;82(3):422–436. doi: 10.1002/jcb.1162. [DOI] [PubMed] [Google Scholar]
- 9.Kim HS, Ingermann AR, Tsubaki J, Twigg SM, Walker GE, Oh Y. Insulin-like growth factor-binding protein 3 induces caspase-dependent apoptosis through a death receptor-mediated pathway in MCF-7 human breast cancer cells. Cancer Research. 2004;64(6):2229–2237. doi: 10.1158/0008-5472.can-03-1675. [DOI] [PubMed] [Google Scholar]
- 10.Xie SP, Pirianov G, Colston KW. Vitamin D analogues suppress IGF-I signalling and promote apoptosis in breast cancer cells. European Journal of Cancer. 1999;35(12):1717–1723. doi: 10.1016/s0959-8049(99)00200-2. [DOI] [PubMed] [Google Scholar]
- 11.Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. Journal of Endocrinology. 2002;175(1):19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- 12.Butt AJ, Firth SM, King MA, Baxter RC. Insulin-like growth factor-binding protein-3 modulates expression of Bax and Bcl-2 and potentiates p53-independent radiation-induced apoptosis in human breast cancer cells. Journal of Biological Chemistry. 2000;275(50):39174–39181. doi: 10.1074/jbc.M908888199. [DOI] [PubMed] [Google Scholar]
- 13.Shahjee H, Bhattacharyya N, Zappala G, Wiench M, Prakash S, Rechler MM. An N-terminal fragment of insulin-like growth factor binding protein-3 (IGFBP-3) induces apoptosis in human prostate cancer cells in an IGF-independent manner. Growth Hormone and IGF Research. 2008;18(3):188–197. doi: 10.1016/j.ghir.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Matilainen M, Malinen M, Saavalainen K, Carlberg C. Regulation of multiple insulin-like growth factor binding protein genes by 1α,25-dihydroxyvitamin D3 . Nucleic Acids Research. 2005;33(17):5521–5532. doi: 10.1093/nar/gki872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malinen M, Ryynänen J, Heinäniemi M, Väisänen S, Carlberg C. Regulation of multiple insulin-like growth factor binding protein genes by 1alpha, 25-dihydroxyvitamin D3 . Nucleic Acids Research. 2011;39(2):502–512. doi: 10.1093/nar/gkq820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schedlich LJ, Muthukaruppan A, O’Han MK, Baxter RC. Insulin-like growth factor binding protein-5 interacts with the vitamin D receptor and modulates the vitamin D response in osteoblasts. Molecular Endocrinology. 2007;21(10):2378–2390. doi: 10.1210/me.2006-0558. [DOI] [PubMed] [Google Scholar]
- 17.Cui J, Ma C, Qiu J, et al. A novel interaction between insulin-like growth factor binding protein-6 and the vitamin D receptor inhibits the role of vitamin D3 in osteoblast differentiation. Molecular and Cellular Endocrinology. 2011;338(1-2):84–92. doi: 10.1016/j.mce.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Colston KW, Hansen CM. Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocrine-Related Cancer. 2002;9(1):45–59. doi: 10.1677/erc.0.0090045. [DOI] [PubMed] [Google Scholar]
- 19.Lee KW, Liu B, Ma L, et al. Cellular internalization of insulin-like growth factor binding protein-3. Distinct endocytic pathways facilitate re-uptake and nuclear localization. Journal of Biological Chemistry. 2004;279(1):469–476. doi: 10.1074/jbc.M307316200. [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharyya N, Pechhold K, Shahjee H, et al. Nonsecreted insulin-like growth factor binding protein-3 (IGFBP-3) can induce apoptosis in human prostate cancer cells by IGF-independent mechanisms without being concentrated in the nucleus. Journal of Biological Chemistry. 2006;281(34):24588–24601. doi: 10.1074/jbc.M509463200. [DOI] [PubMed] [Google Scholar]
- 21.Firth SM, Baxter RC. Characterisation of recombinant glycosylation variants of insulin-like growth factor binding protein-3. Journal of Endocrinology. 1999;160(3):379–387. doi: 10.1677/joe.0.1600379. [DOI] [PubMed] [Google Scholar]
- 22.Coverley JA, Baxter RC. Phosphorylation of insulin-like growth factor binding proteins. Molecular and Cellular Endocrinology. 1997;128(1-2):1–5. doi: 10.1016/s0303-7207(97)04032-x. [DOI] [PubMed] [Google Scholar]
- 23.Narvaez CJ, Welsh J. Role of mitochondria and caspases in vitamin D mediated apoptosis of MCF-7 breast cancer cells. Journal of Biological Chemistry. 276(34):9101–9107. doi: 10.1074/jbc.M006876200. [DOI] [PubMed] [Google Scholar]
- 24.Nickerson T, Huynh H, Pollock M. Insulin-like growth factor binding protein-3 induces apoptosis in MCF7 breast cancer cells. Biochemical Biophysical Research Communication. 1997;237(3):690–693. doi: 10.1006/bbrc.1997.7089. [DOI] [PubMed] [Google Scholar]
- 25.Mathiasen IS, Lademann U, Jäättelä M. Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Research. 1999;59(19):4848–4856. [PubMed] [Google Scholar]
- 26.Mathiasen IS, Sergeev IN, Bastholm L, Elling F, Norman AW, Jäättelä M. Calcium and calpain as key mediators of apoptosis-like death induced by vitamin D compounds in breast cancer cells. Journal of Biological Chemistry. 2002;277(34):30738–30745. doi: 10.1074/jbc.M201558200. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Zhang G, Hendrix LR, Tesh VL, Samuel JE. Coxiella burnetii induces apoptosis during early stage infection via a caspase-independent pathway in human monocytic THP-1 cells. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu M, Ishii H, Ogo N, et al. S-trityl-L-cysteine derivative induces caspase-independent cell death in K562 human chronic myeloid leukemia cell line. Cancer Letters. 2010;298(1):99–106. doi: 10.1016/j.canlet.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Zhu P, Martinvalet D, Chowdhury D, Zhang D, Schlesinger A, Lieberman J. The cytotoxic T lymphocyte protease granzyme A cleaves and inactivates poly(adenosine 5′-diphosphate-ribose) polymerase-1. Blood. 2009;114(6):1205–1216. doi: 10.1182/blood-2008-12-195768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingermann AR, Yang YF, Han J, et al. Identification of a novel cell death receptor mediating IGFBP-3-induced anti-tumor effects in breast and prostate cancer. Journal of Biological Chemistry. 2010;285(39):30233–30246. doi: 10.1074/jbc.M110.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohanraj L, Oh Y. Targeting IGF-I, IGFBPs and IGF-I receptor system in cancer: the current and future in breast cancer therapy. Recent Patents on Anti-Cancer Drug Discovery. 2011;6(2):166–177. doi: 10.2174/157489211795328512. [DOI] [PubMed] [Google Scholar]
- 32.Schedlich LJ, O’Han MK, Leong GM, Baxter RC. Insulin-like growth factor binding protein-3 prevents retinoid receptor heterodimerization: implications for retinoic acid-sensitivity in human breast cancer cells. Biochemical and Biophysical Research Communications. 2004;314(1):83–88. doi: 10.1016/j.bbrc.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 33.Jerome L, Alami N, Belanger S, et al. Recombinant human insulin-like growth factor binding protein 3 inhibits growth of human epidermal growth factor receptor-2-overexpressing breast tumors and potentiates herceptin activity in vivo. Cancer Research. 2006;66(14):7245–7252. doi: 10.1158/0008-5472.CAN-05-3555. [DOI] [PubMed] [Google Scholar]


