Abstract
Objective
Depression is highly comorbid with coronary artery disease. Clinicians face the question of whether patients’ depressive symptoms will improve after coronary artery bypass graft surgery (CABG). The objective of this meta-analysis is to determine the course of depressive symptoms after CABG.
Methods
EMBASE, PubMed, and PsycINFO were searched for studies assessing depression before and after CABG. Meta-analyses were performed for depression at early (1–2 weeks), recovery (>2 weeks to 2 months), mid (>2 months to 6 months), and late (>6 months) postoperative time points. Heterogeneity and publication bias were analyzed.
Results
Thirty-nine studies were included in the meta-analysis. Twelve reported dichotomous outcomes; 18 reported continuous outcomes; and 9 reported both. Risk of depression was increased early (relative risk [RR] = 1.27; 95% confidence interval [CI], 1.01–1.61). There was a significantly decreased risk of depression at recovery (RR = 0.78; 95% CI, 0.67–0.90), mid (RR = 0.64; 95% CI, 0.58–0.70), and late (RR = 0.68; 95% CI, 0.58–0.79) time points without heterogeneity. All studies reporting continuous depression scales had significant heterogeneity.
Conclusions
The risk of depression decreased post-CABG when depression was measured dichotomously. While depression improves overall and remits for some patients after CABG, the majority of patients will not experience remission of depression. Preoperative and postoperative depression monitoring is important.
Keywords: cardiac surgery, coronary artery bypass graft surgery, depression
INTRODUCTION
Coronary artery bypass graft surgery (CABG) is the most common cardiac surgery in the United States, with over 200,000 procedures currently performed a year.1 Depression and coronary artery disease are highly comorbid conditions with estimates of comorbidity from 14% to 47%.2 Many patients undergoing cardiac surgery, especially CABG, suffer from depression, both pre-and postoperatively.2–5 Both preoperative and also postoperative depression predict poor recovery from this procedure. Providers are often faced with the question of whether a patient with major depressive disorder or subclinical depressive symptoms will experience an improvement or worsening in depressive symptoms after CABG. The high comorbidity between coronary artery disease (CAD) and depressive symptoms necessitates an understanding of the effect of CABG on depressive symptoms. Several theories will be discussed.
Preoperative depression is predictive of decreased cardiac symptom relief, quicker return of symptoms, more frequent rehospitalizations, and increased mortality in the immediate postoperative period.3,6 In a prospective study of patients undergoing CABG, Blumenthal and colleagues showed that those patients who were moderately to severely depressed before CABG had a greater than twofold risk of death after surgery as compared to their nondepressed counterparts.2 Additionally, the presence of depression preoperatively is predictive of postoperative depression.7,8 Recent research has shown that depression in CAD patients is often not be detected and treated adequately. 9,10
Postoperative depression is also associated with complicated recovery and poor postoperative outcomes after CABG. Depression after CABG increases both the risk of poor physical and emotional recovery from surgery3,11 and the morbidity and mortality from cardiovascular disease.12 Postoperative depression has been associated with decreased physical function,13 increased risk of cardiovascular events (angina, myocardial infarct, cardiovascular mortality),10,14 and increased mortality.2,15 Postoperative depression is further associated with poor wound healing, increased likelihood of wound infection, and increased risk of cardiac events postoperatively.16 The aim of the present study is conduct a systematic literature review and meta-analysis to examine the course of depressive symptoms after CABG. We hypothesized that depressive symptomatology would improve after surgery due to alleviation of pain, improvement in physical function, and relief of the anticipatory stress of cardiac surgery.
MATERIALS AND METHODS
Search Methodology
EMBASE, PubMed, and PsycINFO were searched. We reviewed studies between October 1, 1995, and June 15, 2011, that were limited to the English language, adults (≥18 years), and human subjects. The cardiac surgery search term was created by the combination of the following medical subject headings: “cardiac surgery, coronary artery bypass graft, CABG, heart surgery, valve replacement OR thoracic surgery” and the depression term included the MeSH terms “major depressive disorder, depression or depression screen or depression scale.”
Abstract Review and Study Selection Criteria
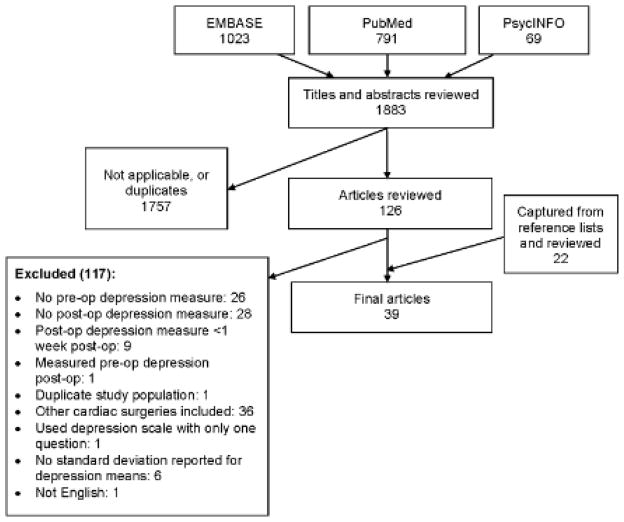
Abstracts using both the cardiac surgery and depression terms were independently reviewed by two authors, and relevant studies were identified for full-text review. Inclusion criteria included prospective studies that measured depression preoperatively and postoperatively (using the same instrument) and that looked specifically at CABG patients. (The search initially included a broad range of cardiac surgeries. We subsequently focused our search on CABG because of the variability in cardiac surgery types and combinations.) We excluded studies examining CABG with valve surgery, where data for CABG alone were not reported. Exclusion criteria included studies that did not specify how depression was assessed, that used single-question depression scales, that did not report postoperative and preoperative depression measurements, that did not report mean and error values for continuous depression measures, that measured postoperative depression less than one week postoperatively, and that measured preoperative depression postoperatively. We did not exclude studies based on their enrollment criteria regarding preexisting mental illness. Additionally, the reference lists of all included studies were manually reviewed for relevant references. Figure 1 is a flow diagram of the article selection process.
Figure 1.
Measurement Variables
Study characteristics, demographic characteristics, and depression measures were abstracted from studies that met inclusion criteria. The mean age of subjects and the percentages of male and female subjects were recorded. We recorded depression at the time points used by the studies preoperatively and postoperatively. Postoperative time points were chosen to reflect a normal course of recovery; recovery to full function after CABG usually takes 1–2 months. Time points were categorized as follows: early (1–2 weeks), recovery (>2 weeks to 2 months), mid (>2 months to 6 months), and late (>6 months). In the analysis of depression expressed as a continuous variable, baseline data were stratified into the proximal baseline assessment (depression assessed ≤1 week preoperatively) and remote baseline assessment groups (depression assessed >1 week preoperatively).
For the studies with dichotomous data, the number of patients identified as depressed and not depressed for each time point measured were abstracted. Additionally, the cutoff on the depression measure used was noted. For those with continuous variables, data points used were the means and standard deviations of scores on the given depression measure for each time point measured by that study. If a study presented both dichotomous and continuous data, both were used in analysis.
Statistical Analysis
Dichotomous outcomes
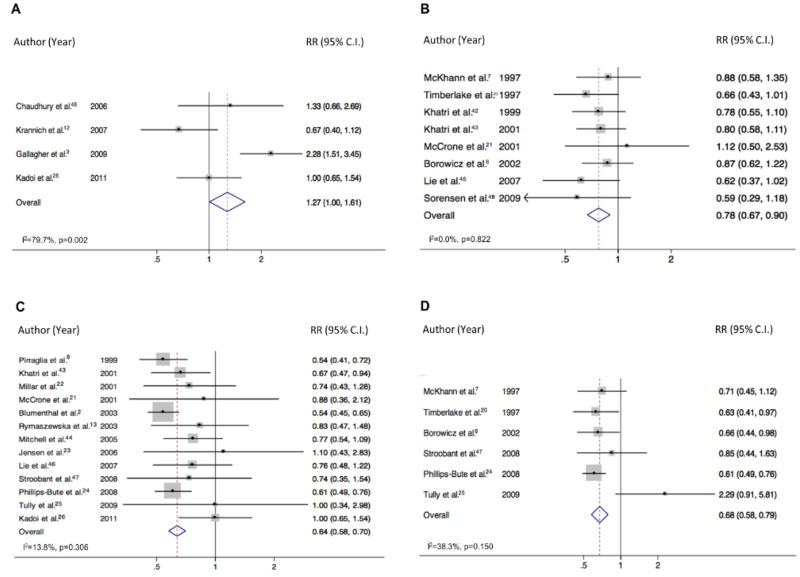
Studies with dichotomous depression outcomes were assembled with the timing of the outcome as the dependent variable and the preoperative assessment as the control variable. A meta-analysis was performed for each of the four postoperative time points. The relative risk of depression at each postoperative time period was calculated and is displayed on the forest plot (Figure 2). Heterogeneity magnitude (I2) and significance was calculated for each meta-analysis.17
Figure 2.
Continuous outcomes
For each of the postoperative time points, we calculated a standardized mean difference (effect size) relative to the mean and standard deviation of the baseline. Heterogeneity magnitude (I2) and significance were calculated. Further analyses were stratified by the timing of the preoperative depression assessment. The proximal baseline assessment included those studies in which depression was evaluated one week or less preoperatively. Remote baseline assessment included those studies in which depression was evaluated greater than one week preoperatively. For these analyses, the overall effect size, 95% confidence interval, and presence of significant heterogeneity were reported at each time point.
Assessment for publication bias
Data were analyzed for publication bias using the methods of Egger18 and Peters19 to assess for small study bias for dichotomous outcomes.
RESULTS
Figure 1 summarizes the search strategy and results. The search identified 1883 abstracts, 126 of which were identified for full text review. The references were also manually searched, yielding an additional 22 articles. 109 studies were excluded. Ultimately 39 studies on depression after CABG fitting the inclusion criteria were identified, including a total of 8,633 patients.
Studies are presented by type of variables used. Table 1 describes all studies (dichotomous only: n = 12; continuous only: n = 18; both dichotomous and continuous: n = 9) including the number of subjects, age, depression measure, and times of follow-up. Table 2 describes the depression measures (n = 10) used by the 39 studies. The Center for Epidemiological Studies Depression Scale and the Hospital Anxiety and Depression Scale, depression rating were the most commonly used tests (n = 13 for each).
Table 1.
Characteristics of 39 Eligible Studiesa
| Type of study (&RCT intervention) | n (pre-op/final post-op) | Post-op measurement times | Depression measure & cutoff | Mean age in years (SD) | % women (n) | CPB used?b | |
|---|---|---|---|---|---|---|---|
| Dichotomous | |||||||
| McKhann et al. (1997)7 | Cohort | 124/124 | 1 month, 1 year | CES-D > 16 | 63.0 (NR) | 21.3 (27) | NR |
| Timberlake et al. (1997)20 | Cohort | 103/103 | 2 months, 1 year | BDI ≥ 9 | 56.0 (7.9) | 9.9 (12) | Yes |
| Pirraglia et al. (1999)8 | Cohort | 218/218 | 6 months | CES-D ≥ 16 | 65.3 (9.3) | 18.8 (41) | Yes |
| McCrone et al. (2001)21 | Cohort | 31/31 | 8 weeks, 3 months | CES-D ≥ 16 | 70.0 (8.6) | 38.7 (12) | NR |
| Millar et al. (2001)22 | Cohort | 81/81 | 6 months | BDI ≥ 9 | 60.9 (9.4) | 21 (17) | Yes |
| Borowicz et al. (2002)6 | Cohort | 172/132 | 1 month, 1 year | CES-D ≥ 16 | 63.4 (NR) | 22.1 (38) | NR |
| Blumenthal et al. (2003)2 | Cohort | 817/490 | 6 months | CES-D ≥ 16 | 61.0 (10.2) | 27.1 (221) | NR |
| Rymaszewska et al. (2003)13 | Cohort | 56/53 | 3 months | BDI (cutoff NR) | 58.6 (NR) | 30.2 (16) | Yes |
| Jensen et al. (2006)23 | RCT (on-pump/off-pump) | 120/109 | 3 months | MDI > 2.5 | 75.5 (4.5) | 40 (48) | Bothb |
| Phillips-Bute et al. (2008)24 | Cohort | 427/411 | 6 months, 1 year | CES-D ≥ 16 | 61.0 (10.9) | 30 (128) | Yes |
| Tully et al. (2009)25 | Cohort | 86/75 | 6 months, 5 years | DASS ≥ 10 | 65.1 (9.9) | 26.7 (23) | Yes |
| Kadoi et al. (2011)26 | Cohort | 90/90 | 1 week, 6 months | BDI ≥ 10 | 65.0 (9.0) | 24.4 (22) | Yes |
| Continuous | |||||||
| Duits et al. (1999)4 | Cohort | 270/217 | 1week, 6 months | HADS-D | 60.8 (8.8) | 18.9 (41) | NR |
| Edell-Gustafsson & Hetta (1999)27 | Cohort | 38/38 | 1 & 6 months | Zung SDS | 61.3 (5.0) | 0 (0) | NR |
| Keresztes et al. (2003)28 | Cohort | 80/80 | 1 & 3 months | POMS-D | 63.3 (11.5) | 50 (40) | NR |
| Lindquist et al. (2003)29 | Cohort | 674/600 | 6 weeks, 6 months, 1 year | CES-D | 63.6 (9.4) | 39.9 (269) | NR |
| Knipp et al. (2004)30 | Cohort | 35/29 | 3 months | HADS-D | 67.6 (8.7) | 17.2 (5) | Yes |
| Phillips-Bute et al. (2006)31 | Cohort | 732/551 | 6 weeks, 1 year | CES-D | 62.3 (11.0) | 39.2 (216) | Yes |
| Ruiz et al. (2006)32 | Cohort | 111/97 | 18 months | CES D-10 | 61.1 (10.3) | 0 (0) | NR |
| Sorlie et al. (2006)33 | RCT (educational intervention/control) | 109/87 | 6 weeks, 6 months, 1 year | Zung SDS | 58.3 (6.3) | 11.9 (13) | NR |
| Szalma et al. (2006)34 | RCT (piracetem/placebo) | 109/88 | 6 weeks | BDI | 55.9 (5.6) | 17.4 (19) | NR |
| Bay et al. (2007)35 | RCT (religious intervention/control) | 170/170 | 1 & 6 months | HADS-D | 64.0 (NR) | 24.7 (42) | NR |
| Lopez et al. (2007)36 | Cohort | 68/68 | 1 week, 6 months | CES-D | 63.9 (10.1) | 33.8 (23) | Both |
| Murphy et al. (2008)5 | Cohort | 180/119 | 2 & 6 months | HADS-D | 65.6 (9.8) | 21.7 (39) | NR |
| Sandau et al. (2008)37 | Cohort | 54/54 | 3 months | CES-D | 65.2 (9.3) | 21.9 (14) | Both |
| Azzopardi & Lee (2009)38 | Cohort | 48/48 | 2 years | BDI | 66.6 (9.9) | 14.6 (7) | NR |
| Elliott et al. (2010)39 | Cohort | 174/117 | 2 & 6 months | POMS-D | 65.5 (9.8) | 21.3 (37) | NR |
| Kozora et al. (2010)40 | RCT (on-pump/off- pump) | 1801/1156 | 1 year | BDI | 62.0 (8.2) | 0.6 (7) | Both |
| van Mastrigt et al. (2010)41 | RCT (short-stay intensive care treatment/control) | 406/361 | 1 month, 1 year | BDI | 61.9 (12.2) | 19.2 (78) | Both |
| Nemati & Astaneh (2011)11 | Cohort | 71/71 | 1 month | HADS-D | 59.9 (8.7) | 26.8 (19) | NR |
| Dichotomous & continuous | |||||||
| Khatri et al. (1999)42 | Cohort | 170/170 | 6 weeks | CES-D | 61.0 (10.0) | 25.3 (43) | NR |
| Khatri et al. (2001)43 | RCT (hypothermic/normothermic) | 226/208 | 6 weeks, 6 months | CES-D >16 | 61.6 (12.7) | 24.8 (56) | Yes |
| Mitchell et al. (2005)44 | Cohort | 123/120 | 2 months | BDI ≥10 | 63.3 (10.2) | 44.2 (53) | NR |
| Chaudhury et al. (2006)45 | Cohort | 30/30 | 1 week | HADS-D (cutoff NR) | 60.0 (5.5) | 10 (3) | NR |
| Krannich et al. (2007)12 | Cohort | 97/97 | 10 days | HADS-D ≥ 8 | 65.0 (8.5) | 18.6 (18) | NR |
| Lie et al. (2007)46 | RCT (psychological support/control) | 203/185 | 6 weeks, 6 months | HADS-D ≥ 8 | 62.0 (NR) | 10.3 (9) | NR |
| Stroobant & Vingerhoets (2008)47 | Cohort | 53/43 | 6 months, 3–5 years | BDI-CA ≥ 4 | 59.4 (7.2) | 7.5 (4) | Both |
| Gallagher & McKinley (2009)3 | Cohort | 155/126 | 2 weeks | HADS-D ≥ 8 | 66.3 (10.7) | 26.5 (41) | NR |
| Sorensen & Wang (2009)48 | Cohort | 70/63 | 6 weeks | GDS ≥ 5 | 72.0 (5.7) | 34.3 (24) | NR |
BDI, Beck Depression Inventory; BDI-CA, Beck Depression Inventory (cognitive-affective subscale); CES-D, Center for Epidemiologic Studies Depression Scale; CES-D-10, Center for Epidemiologic Studies Depression Scale (10-item version); CPB, cardiopulmonary bypass; DASS, Depression Anxiety Stress Scale (depression subscale); GDS, Geriatric Depression Scale; HADS-D, Hospital Anxiety and Depression Scale (depression subscale); MDI, Major Depression Inventory; NR, not reported; POMS-D, Profile of Mood States Depression Scale; RCT, randomized, controlled trial; SD, standard deviation; Zung SDS, Zung Self-Rating Depression Scale.
Dichotomous only: n = 12; continuous only: n = 18; dichotomous and continuous: n = 9.
Yes refers to the use of CPB (on-pump). Both includes surgeries with (on-pump) and without (off-pump) CPB.
Table 2.
The Ten Depression Measures Used
| Depression measure | Scale | Significance of score | Continuous studies (total n = 27) | Dichotomous studies (total n = 21) | |||
|---|---|---|---|---|---|---|---|
| Studies (n) | Studies | Studies (n) | Studies | Cutoffs used: studies (n) using the cutoff | |||
| Beck Depression Inventory34 | 0–63 | 0–9: normal 10–15: mild depression 16–19: mild to moderate depression 20–29: moderate to severe depression 30–63: severe depression |
5 | Mitchell (2005)44 Szalma et al. (2006)34 Azzopardi (2009)38 Kozora (2010)40 van Mastrigt (2010)41 |
5 | Timberlake (1997)20 Millar (2001)22 Rymaszewska (2003)13 Mitchell (2005)44 Kadoi (2011)26 |
≥ 9:2 ≥10:3 NR:1 |
| Beck Depression Inventory (cognitive-affective subscale)47 | 0–39 | 0–3: normal 4–6: mild depression >7: moderate depression |
1 | Stroobant (2008)47 | 1 | Stroobant (2008)47 | ≥ 4:1 |
| Center for Epidemiologic Studies Depression Scale49 | 0–60 | Higher scores represent worse depressive symptoms | 6 | Khatri (1999)42 Khatri (2001)43 Lindquist (2003)29 Phillips-Bute (2006)31 Lopez (2007)36 Sandau (2008)37 |
8 | McKhann (1997)7 Khatri (1999)42 Pirraglia (1999)8 Khatri (2001)43 McCrone (2001)21 Borowicz (2002)6 Blumenthal (2003)2 Phillips-Bute (2008)24 |
>16:3 ≥16:5 |
| Center for Epidemiologic Studies Depression Scale (10-item version)32,49 | 0–30 | Higher scores represent worse depressive symptoms | 1 | Ruiz (2006)32 | 0 | ||
| Depression Anxiety Stress Scale (depression subscale)25 | 0–42 | Higher scores represent worse depressive symptoms | 0 | 1 | Tully (2009)25 | ≥ 10:1 | |
| Geriatric Depression Scale (15-item)50 | 0–15 | ≤10: normal 11–20: mild depression ≥21: moderate to severe depression |
1 | Mui (1996)50 | 1 | Mui (1996)50 | ≥ 5:1 |
| Hospital Anxiety and Depression Scale (depression subscale)3 | 0–21 | 0–7: low levels of depression 8–10: borderline cases ≥10: clinical cases |
9 | Duits (1999)4 Knipp (2004)30 Chaudhury (2006)45 Krannich (2007)12 Lie (2007)46 Bay (2008)35 Murphy (2008)5 Gallagher (2009)3 Nemati (2011)11 |
4 | Chaudhury (2006)45 Krannich (2007)12 Lie (2007)46 Gallagher (2009)3 |
≥ 8:3 NR:1 |
| Major Depression Inventory23 | 0–50 | 20–24: mild depression 25–29: moderate depression ≥ 30: severe depression |
0 | 1 | Jensen (2006)23 | >2.5:1 | |
| Profile of Mood States Depression Scale28 | 0–60 | Higher scores represent worse depressive symptoms | 2 | Keresztes (2003)28 Elliott (2010)39 |
0 | ||
| Zung Self-Rating Depression Scale51 | 25–100 | 25–49: normal 50–59: mild depression 60–69: moderate depression ≥ 70: severe depression |
2 | Edell-Gustafsson (1999)27 Sørlie (2007)33 |
0 | ||
NR, not reported.
Table 3 displays the results of the meta-analyses for both the dichotomous and continuous data, as well as the results of the heterogeneity and publication bias analyses for each time point. Thirty percent of subjects were depressed preoperatively, and 21.6% were depressed at the latest (>6 months) postoperative time point.
TABLE 3.
Summary of Depressive Symptoms Postoperatively by Type of Study
| Postoperative time points | ||||
|---|---|---|---|---|
| Early (1 to 2 weeks) | Recovery (from 2 weeks to 2 months) | Mid (from 2 to 6 months) | Late (over 6 months) | |
| Dichotomous studies (n = 21) | ||||
| Overall baseline assessment (n = 21) | ||||
| % depressed (median)a | 35.5% | 24% | 22% | 21.6% |
| % depressed (range) | 18%–45% | 11%–29% | 7%–31% | 16%–26% |
| Relative risk | 1.27 | 0.78 | 0.64 | 0.68 |
| 95% CI | 1.01, 1.61 | 0.67, 0.90 | 0.58, 0.70 | 0.58, 0.79 |
| Number of studiesb | 4 | 8 | 13 | 6 |
| Heterogeneity | Yes | No | No | No |
| Publication bias | No | No | Yes | No |
| Continuous studies (n = 27) | ||||
| Overall baseline assessment (n = 27) | ||||
| Standard mean difference | 0.21 | − 0.19 | − 0.41 | − 0.21 |
| 95% CI | 0.09, 0.33 | − 0.24, −0.14 | −0.47, −0.35 | −0.26, −0.16 |
| Number of studiesb | 5 | 15 | 15 | 8 |
| Heterogeneity | Yes | Yes | Yes | Yes |
| Publication bias | No | No | No | No |
| Proximal baseline assessment (n = 10) | ||||
| Standard mean difference | 0.24 | − 0.17 | − 0.48 | − 0.20 |
| 95% CI | 0.04, 0.45 | − 0.24, −0.09 | − 0.57, −0.39 | − 0.26, −0.15 |
| Number of studiesb | 3 | 4 | 5 | 3 |
| Heterogeneity | Yes | Yes | Yes | Yes |
| Publication bias | Yes | No | No | No |
| Remote baseline assessment (n = 6) | ||||
| Standard mean difference | − 0.08 | − 0.34 | − 0.35 | − 0.25 |
| 95% CI | − 0.26, 1.00 | − 0.48, 0.19 | − 0.46, −0.24 | − 0.52, 0.03 |
| Number of studiesb | 1 | 4 | 4 | 1 |
| Heterogeneity | NA | No | No | NA |
| Publication bias | NA | No | No | NA |
CI, Confidence Interval; NA, not enough data available for analysis.
Median percentage depressed preoperatively was 30%, with a range of 7% to 43%.
Since some studies take measurements at more than one time point, the number of studies adds up to more than the number in each broader category.
Figure 2 shows the Forest Plots from the meta-analysis of the dichotomous data at each of the four postoperative time points. The early time point (panel A) showed an increased risk of depression relative to baseline (relative risk [RR] = 1.27; 95% confidence interval [CI], 1.01–1.61) but showed significant heterogeneity between studies (I2 = 79.70%; p for heterogeneity < .001). By contrast, the recovery time point (panel B) showed a significant decreased risk of depression (RR = 0.78; 95% CI, 0.67–0.90) and no between-study heterogeneity (I2 = 0.00%; p for heterogeneity = 0.82). The mid time point (panel C) showed a more pronounced decrease in depression risk post-surgery (RR = 0.64; 95% CI, 0.58–0.70), also without heterogeneity (I2 = 13.80%; p for heterogeneity = .31). Analysis of the late postoperative time point (panel D) also showed a reduction in depression (RR = 0.68; 95% CI, 0.58–0.79) without heterogeneity (I2 = 38.30%; p for heterogeneity = 0.15), indicating that this decreased risk of depression was sustained throughout the long-term postoperative period.
Analysis of the continuous data yielded significant heterogeneity across all time points. In the stratified data, the proximal baseline assessment group also showed significant heterogeneity across all time points; thus, these data cannot be considered statistically valid. Upon stratification, there was a decrease in standardized mean difference (SMD) for the remote baseline assessment group without heterogeneity at all time points: early (SMD = −0.08; 95% CI, −0.26 to 1.00); recovery (SMD = −0.34; 95% CI, −0.48 to 0.19); mid (SMD = −0.35; 95% CI, −0.46 to −0.24), and late (SMD = −0.25; 95% CI, − 0.52 to 0.03).
Only one time point in the dichotomous data (mid) and one in the stratified continuous data (early, in the proximal baseline assessment group) demonstrated publication bias.
DISCUSSION
This study found that depressive depression is prevalent in patients undergoing CABG, both before and after the procedure. The overall risk of being depressed after CABG is decreased, though greater than 20% of those who undergo CABG are depressed in the months afterward, after the immediate postoperative period has passed. This conclusion was drawn from a systematic synthesis and meta-analysis of data on depression and depressive symptoms after CABG from 39 studies.
These findings have clinical application in multiple areas, including psychiatry, cardiac surgery, cardiology, primary care, and research. Depression is highly comorbid with coronary artery disease. Clinicians frequently face the question of whether a patient’s depressive symptoms are likely to change after CABG. The findings of this study suggest that significant improvement in depressive symptoms is experienced by nearly one-third of those preoperatively depressed but that a significant portion (approximately one-fifth) of those who have undergone CABG remain depressed or develop new depression.
The relationship between cardiovascular disease, especially CAD, and depression has been widely examined. There is likely a reciprocal relationship between the two. The association between depression and poor outcomes in cardiovascular disease is well established.3,11,45 Cardiovascular disease contributes to depression according to the vascular-depression hypothesis that thromboembolism and hypotension from vascular disease reduce perfusion to brain areas associated with depression—in particular, the frontal-subcortical circuits and hippocampus.
While this study found overall improvement in depressive symptoms after CABG, for the majority of patients, depression persists after the surgery.2,8,20,22 Thus, both preoperative and postoperative assessments of depression are critical in the CABG patient. Preoperative assessment is important because preoperative depression is predictive of the ability to weather the stress of major surgery and may therefore compromise effective postoperative recovery.3 More specifically, preoperative assessment of depression enables the identification of patients at risk for delirium, poor recovery, and subsequent depression; postoperative interventions and depression monitoring can therefore focus on these patients. Note, too, that because preoperative depression is associated with postoperative mortality,2 study dropout due to mortality may result in a more positive picture of postoperative depression than is actually the case. Regular postoperative assessment is needed to identify and treat depressed patients.
The current study highlights the importance of the timing of depression measures before and after CABG in assessing clinically meaningful mood disturbance. As the stratified continuous data showed, measuring preoperative depression close to the time of surgery (≤1 week prior) may yield results that do not reflect clinically meaningful mood disorders. A depression measure taken too close to the point of surgery may be reflecting anticipation of impending surgery and a worsening of physical symptoms.4 Similarly, as shown in the dichotomous data, a measurement taken in the two weeks after surgery may reflect the known consequences of surgery and perioperative care (e.g., pain, poor sleep, complications) rather than a mood disorder. If preoperative depression is assessed in sufficient time before (>1 week) and after (>2 weeks) surgery, this evaluation will yield more clinically useful information and generate more appropriate interventions.
Research on this topic has been intense in recent years. A major strength of the present meta-analysis is the large number of studies available for inclusion. Another strength is the ability to break down the data into discrete postoperative time points, which allowed the course of depressive symptoms during recovery to be measured precisely. Depression was analyzed as both a continuous and a dichotomous variable, allowing the examination of fluctuation in depressive symptoms before and after CABG. Heterogeneity and publication bias were also systematically measured. This analysis yielded dichotomous data that displayed heterogeneity only at the earliest time point and publication bias only at one of the four time points.
This study has several limitations. Studies included in our analysis measured depressive symptoms using clinical assessment tools—most frequently, the Center for Epidemiological Studies Depression Scale and the Hospital Anxiety and Depression Scale, depression rating—rather than diagnosing depression through clinical interviews. Additionally, to be included in this meta-analysis, studies needed to assess depression both before and after surgery. This condition excluded many studies that examined depressive symptoms only before CABG, and it also likely limited the population included in the meta-analysis to those undergoing planned CABG (to allow sufficient time preoperatively to assess depression). Relatively few women were included, though this limitation likely reflects the population receiving non-emergent CABG. Our study was also limited by not knowing the patients’ indications for CABG, patients’ comorbid medical and psychiatric conditions, and the characteristics of the hospitalizations (e.g., length of stay). We were also unable to analyze depression risk by age, sex, or surgical and other treatment characteristics because these variables were not consistently reported.
The population included in this study was heterogeneous in terms of preexisting mental illness. Some studies excluded patients who had a history of psychiatric illness, whereas others did not assess or report preoperative diagnosis of mental illness. Likewise, none of the studies reported postoperative treatment for depression. It is not known whether treatment specifically for depression (e.g., psychotherapy or pharmacotherapy) contributed to the improvement in depressive symptoms that was observed. A recent randomized, cross-sectional survey of U.S. cardiovascular physicians revealed that nearly three-fourths of them ask less than half their patients about depression and that over three-fourths of them (79%) of them do not use a standard screening tool. 9 Just under half stated that they treat depression.9 According to a nationally representative study on the prevalence and correlates of major depressive disorder in a general adult population, its 12-month prevalence was 16.2%, and 21.7% of those with major depressive disorder were adequately treated for their depression.10 This percentage of adequate treatment, though low, could explain some of the improvement in depressive symptoms observed after CABG. This same low percentage of adequate treatment also highlights, however, the need for depression screening and treatment in the especially vulnerable population of those with CAD.
Other significant limitations of this meta-analysis are that it included both interventional and observational studies and that the quality of included studies was not systematically assessed.
Finally, analysis of the continuous data was limited by the heterogeneity found across all time points in the overall baseline assessment data. Though sub-analysis yielded a remote baseline assessment group without heterogeneity, assessing depression greater than one week prior to surgery is more meaningful than doing so shortly before surgery.
This meta-analysis found significant depression both before and also after CABG. Systematic screening for depression in the period both before and after this procedure is crucial. Identifying depression in CABG patients is important in view of the high comorbidity of depression in those with coronary artery disease, the negative effect of depression on postoperative recovery, morbidity, and mortality, and the treatability of depression.
Acknowledgments
Supported by American Federation for Aging Research, Boston MSTAR, and National Institute of Health (grant no. AG038027-01) (Ms. Bader and Dr. Rudolph); a Harvard Medical School Kaplan Depression Fellowship (Dr. Azar); and a VA Rehabilitation Career Development Award (Dr. Rudolph).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumenthal JA, Lett HS, Babyak MA, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet. 2003;362:604–9. doi: 10.1016/S0140-6736(03)14190-6. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher R, McKinley S. Anxiety, depression and perceived control in patients having coronary artery bypass grafts. J Adv Nurs. 2009;65:2386–96. doi: 10.1111/j.1365-2648.2009.05101.x. [DOI] [PubMed] [Google Scholar]
- 4.Duits AA, Duivenvoorden HJ, Boeke S, et al. A structural modeling analysis of anxiety and depression in patients undergoing coronary artery bypass graft surgery: a model generating approach. J Psychosom Res. 1999;46:187–200. doi: 10.1016/s0022-3999(98)00046-4. [DOI] [PubMed] [Google Scholar]
- 5.Murphy BM, Elliott PC, Higgins RO, et al. Anxiety and depression after coronary artery bypass graft surgery: most get better, some get worse. Eur J Cardiovasc Prev Rehabil. 2008;15:434–40. doi: 10.1097/HJR.0b013e3282fbc945. [DOI] [PubMed] [Google Scholar]
- 6.Borowicz L, Jr, Royall R, Grega M, Selnes O, Lyketsos C, McKhann G. Depression and cardiac morbidity 5 years after coronary artery bypass surgery. Psychosomatics. 2002;43:464–71. doi: 10.1176/appi.psy.43.6.464. [DOI] [PubMed] [Google Scholar]
- 7.McKhann GM, Borowicz LM, Goldsborough MA, Enger C, Selnes OA. Depression and cognitive decline after coronary artery bypass grafting. Lancet. 1997;349:1282–4. doi: 10.1016/S0140-6736(96)09466-4. [DOI] [PubMed] [Google Scholar]
- 8.Pirraglia PA, Peterson JC, Williams-Russo P, Gorkin L, Charlson ME. Depressive symptomatology in coronary artery bypass graft surgery patients. Int J Geriatr Psychiatry. 1999;14:668–80. doi: 10.1002/(sici)1099-1166(199908)14:8<668::aid-gps988>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Khawaja IM, Westermeyer JJ, Gajwani P, et al. Depression and coronary artery disease: the association, mechanisms and therapeutic implications. Psychiatry. 2009;6:38–51. [PMC free article] [PubMed] [Google Scholar]
- 10.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the national comorbidity survey replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 11.Nemati MH, Astaneh B. The impact of coronary artery bypass graft surgery on depression and anxiety. J Cardiovasc Med. 2011;12:401–4. doi: 10.2459/JCM.0b013e32834358e9. [DOI] [PubMed] [Google Scholar]
- 12.Krannich J-H, Weyers P, Lueger S, Herzog M, Bohrer T, Elert O. Presence of depression and anxiety before and after coronary artery bypass graft surgery and their relationship to age. BMC Psychiatry. 2007;7:47. doi: 10.1186/1471-244X-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rymaszewska J, Kiejna A, Hadrys T. Depression and anxiety in coronary artery bypass grafting patients. Eur Psychiatry. 2003;18:155–60. doi: 10.1016/s0924-9338(03)00052-x. [DOI] [PubMed] [Google Scholar]
- 14.Rafanelli C, Milaneschi Y, Roncuzzi R. Minor depression as a short-term risk factor in outpatients with congestive heart failure. Psychosomatics. 2009;50:493–9. doi: 10.1176/appi.psy.50.5.493. [DOI] [PubMed] [Google Scholar]
- 15.Thomas AJ, Kalaria RN, O’Brien JT. Depression and vascular disease: what is the relationship? J Affect Disord. 2004;79:81–95. doi: 10.1016/S0165-0327(02)00349-X. [DOI] [PubMed] [Google Scholar]
- 16.Doering L, Moser D, Lemankiewicz W, Luper C, Khan S. Depression, healing and recovery from coronary artery bypass surgery. Am J Crit Care. 2005;4:316–24. [PubMed] [Google Scholar]
- 17.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–80. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 20.Timberlake N, Klinger L, Smith P, et al. Incidence and patterns of depression following coronary artery bypass graft surgery. J Psychosom Res. 1997;43:197–207. doi: 10.1016/s0022-3999(96)00002-5. [DOI] [PubMed] [Google Scholar]
- 21.McCrone S, Lenz E, Tarzian A, Perkins S. Anxiety and depression: incidence and patterns in patients after coronary artery bypass graft surgery. Appl Nurs Res. 2001;14:155–64. doi: 10.1053/apnr.2001.24414. [DOI] [PubMed] [Google Scholar]
- 22.Millar K, Asbury AJ, Murray GD. Pre-existing cognitive impairment as a factor influencing outcome after cardiac surgery. Br J Anaesth. 2001;86:63–7. doi: 10.1093/bja/86.1.63. [DOI] [PubMed] [Google Scholar]
- 23.Jensen BØ, Hughes P, Rasmussen LS, Pedersen PU, Steinbrüchel DA. Health-related quality of life following off-pump versus on-pump coronary artery bypass grafting in elderly moderate to high-risk patients: a randomized trial. Eur J Cardiothorac Surg. 2006;30:294–9. doi: 10.1016/j.ejcts.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Phillips-Bute B, Mathew JP, Blumenthal JA, et al. Relationship of genetic variability and depressive symptoms to adverse events after coronary artery bypass graft surgery. Psychosom Med. 2008;70:953–9. doi: 10.1097/PSY.0b013e318187aee6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tully PJ, Baker RA, Knight JL, Turnbull DA, Winefield HR. Neuropsychological function 5 years after cardiac surgery and the effect of psychological distress. Arch Clin Neuropsychol. 2009;24:741–51. doi: 10.1093/arclin/acp082. [DOI] [PubMed] [Google Scholar]
- 26.Kadoi Y, Kawauchi C, Ide M, et al. Preoperative depression is a risk factor for postoperative short-term and long-term cognitive dysfunction in patients with diabetes mellitus. J Anaesth. 2011;25:10–7. doi: 10.1007/s00540-010-1072-5. [DOI] [PubMed] [Google Scholar]
- 27.Edell-Gustafsson UM, Hetta JE. Anxiety, depression and sleep in male patients undergoing coronary artery bypass surgery. Scand J Caring Sci. 1999;13:137–43. [PubMed] [Google Scholar]
- 28.Keresztes PA, Merritt SL, Holm K, Penckofer S, Patel M. The coronary artery bypass experience: gender differences. Heart Lung. 2003;32:308–19. doi: 10.1016/s0147-9563(03)00101-8. [DOI] [PubMed] [Google Scholar]
- 29.Lindquist R, Dupuis G, Terrin ML, et al. Comparison of health-related quality-of-life outcomes of men and women after coronary artery bypass surgery through 1 year: findings from the POST CABG Biobehavioral Study. Am Heart J. 2003;146:1038–44. doi: 10.1016/S0002-8703(03)00451-4. [DOI] [PubMed] [Google Scholar]
- 30.Knipp SC, Matatko N, Wilhelm H, et al. Evaluation of brain injury after coronary artery bypass grafting. A prospective study using neuropsychological assessment and diffusion-weighted magnetic resonance imaging. Eur J Cardiothorac Surg. 2004;25:791–800. doi: 10.1016/j.ejcts.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Phillips-Bute B, Mathew JP, Blumenthal JA, et al. Association of neurocognitive function and quality of life 1 year after coronary artery bypass graft (CABG) surgery. Psychosom Med. 2006;68:369–75. doi: 10.1097/01.psy.0000221272.77984.e2. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz J, Matthews K, Scheier M, Schulz R. Does who you marry matter for your health? Influence of patients’ and spouses’ personality on their partners’ psychological well-being following coronary artery bypass surgery. J Pers Soc Psychol. 2006;91:255–67. doi: 10.1037/0022-3514.91.2.255. [DOI] [PubMed] [Google Scholar]
- 33.Sørlie T, Busund R, Sexton J, Sexton H, Sørlie D. Video information combined with individualized information sessions: effects upon emotional well-being following coronary artery bypass surgery—a randomized trial. Patient Educ Couns. 2007;65:180–8. doi: 10.1016/j.pec.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Szalma I, Kiss Á, Kardos L, et al. Piracetam prevents cognitive decline in coronary artery bypass: a randomized trial versus placebo. Ann Thorac Surg. 2006;82:1430–5. doi: 10.1016/j.athoracsur.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Bay P, Beckman D, Trippi J, Gunderman R, Terry C. The effect of pastoral care services on anxiety, depression, hope, religious coping, and religious problem solving styles: a randomized controlled study. J Relig Health. 2008;47:57–69. doi: 10.1007/s10943-007-9131-4. [DOI] [PubMed] [Google Scholar]
- 36.Lopez V, Sek Ying C, Poon C-Y, Wai Y. Physical, psychological and social recovery patterns after coronary artery bypass graft surgery: a prospective repeated measures questionnaire survey. Int J Nurs Stud. 2007;44:1304–15. doi: 10.1016/j.ijnurstu.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Sandau KE, Lindquist RA, Treat-Jacobson D, Savik K. Health-related quality of life and subjective neurocognitive function three months after coronary artery bypass graft surgery. Heart Lung. 2008;37:161–72. doi: 10.1016/j.hrtlng.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Azzopardi S, Lee G. Health-related quality of life 2 years after coronary artery bypass graft surgery. J Cardiovasc Nurs. 2009;24:232–40. doi: 10.1097/JCN.0b013e31819b2125. [DOI] [PubMed] [Google Scholar]
- 39.Elliott PC, Murphy BM, Oster KA, Le Grande MR, Higgins RO, Worcester MUC. Changes in mood states after coronary artery bypass graft surgery. Eur J Cardiovasc Nurs. 2010;9:188–94. doi: 10.1016/j.ejcnurse.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Kozora E, Kongs S, Collins JF, et al. Cognitive outcomes after on- versus off-pump coronary artery bypass surgery. Ann Thorac Surg. 2010;90:1134–41. doi: 10.1016/j.athoracsur.2010.05.076. [DOI] [PubMed] [Google Scholar]
- 41.van Mastrigt G, Joore M, Nieman F, Severens J, Maessen J. Health-related quality of life after fast-track treatment results from a randomized controlled clinical equivalence trial. Qual Life Res. 2010;19:631–42. doi: 10.1007/s11136-010-9625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khatri P, Babyak M, Clancy C, et al. Perception of cognitive function in older adults following coronary artery bypass surgery. Health Psychol. 1999;18:301–6. doi: 10.1037//0278-6133.18.3.301. [DOI] [PubMed] [Google Scholar]
- 43.Khatri P, Babyak M, Croughwell ND, et al. Temperature during coronary artery bypass surgery affects quality of life. Ann Thorac Surg. 2001;71:110–6. doi: 10.1016/s0003-4975(00)02350-x. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell RHB, Robertson E, Harvey PJ, et al. Sex differences in depression after coronary artery bypass graft surgery. Am Heart J. 2005;150:1017–25. doi: 10.1016/j.ahj.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Chaudhury CS, Sharma CS, Pawar SCA, et al. Psychological correlates of outcome after coronary artery bypass graft. Armed Forces Med J India. 2006;62:220–3. doi: 10.1016/S0377-1237(06)80004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lie I, Arnesen H, Sandvik L, Hamilton G, Bunch EH. Effects of a home-based intervention program on anxiety and depression 6 months after coronary artery bypass grafting: a randomized controlled trial. J Psychosom Res. 2007;62:411–8. doi: 10.1016/j.jpsychores.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Stroobant N, Vingerhoets G. Depression, anxiety, and neuropsychological performance in coronary artery bypass graft patients: a follow-up study. Psychosomatics. 2008;49:326–31. doi: 10.1176/appi.psy.49.4.326. [DOI] [PubMed] [Google Scholar]
- 48.Sorensen EA, Wang F. Social support, depression, functional status, and gender differences in older adults undergoing first-time coronary artery bypass graft surgery. Heart Lung. 2009;38:306–17. doi: 10.1016/j.hrtlng.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Radloff LS. The CES-D scale. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 50.Mui A. Geriatric Depression Scale as a community screening instrument for elderly Chinese immigrants. Int Psychogeriatr. 1996;8:445–58. doi: 10.1017/s1041610296002803. [DOI] [PubMed] [Google Scholar]
- 51.Zung W. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]