Abstract
Flavan 3-ols, a type of polyphenolic substance, are distributed in a number of plant foods. Of these foods, chocolate is very rich in flavan 3-ols as flavan 3-ol monomers, (+)-catechin and (−)-epicatechin, and the oligomers as procyanidins. There is evidence that cacao products containing flavan 3-ols have the potential to contribute to the risk reduction of cardiometabolic disorders according to recent epidemiological or intervention studies. This review focuses on recent advances in research on the ability of flavan 3-ols to reduce the risk of cardiovascular disease as a result of improving metabolic syndrome risk factors and these mechanisms.
Keywords: flavan 3-ols, chocolate, cardiovascular diseases, metabolic syndrome, risk factors
Introduction
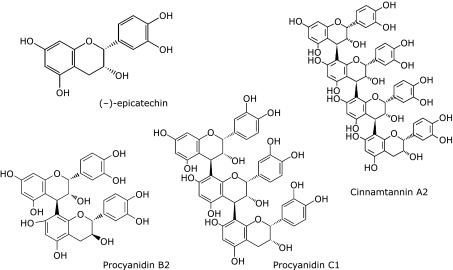
Flavan 3-ols, a type of polyphenolic substance, are distributed in a number of plant foods and supplements such as cacao beans, red wine, beer, berries, apples, black soy bean and French maritime pine bark. Of these foods, chocolate is the most abundant flavan 3-ols containing food. As shown in Fig. 1, these include the flavan 3-ol monomers, (+)-catechin and (−)-epicatechin, and the oligomers, B-type flavan 3-ols, such as procyanidin B2 (dimer), procyanidin C1 (trimer), and cinnamtannin A2 (tetramer) that are linked by C4–C8 bonds.(1–3) It has been reported that chocolate contains oligomers ranging from dimers to decamer flavan 3-ols.(4) Recent studies have suggested that chocolate or flavan 3-ols have a positive influence on human health, due to antioxidant,(5,6) anti-inflammatory,(7,8) and anti-thrombotic effects.(9) There is also evidence that cacao products containing flavan 3-ols have the potential to contribute to the prevention of cardiometabolic disorders.(10) This review focuses on recent advances in research on the ability of flavan 3-ols to reduce the risk of cardiovascular disease as a result of improving metabolic syndrome risk factors.
Fig. 1.
Inverse Association of Chocolate Intake and the Risk of Developing Cardiovascular Diseases
The Kuna, an indigenous group who lives predominantly on small islands off the coast of Panama consume a large amount of natural cocoa drinks, and are nearly free of hypertension and cardiovascular disease. In contrast, Kuna who migrate to Panama urban sites lose this advantage, as they are no longer able to maintain their habit of drinking cocoa.(11) Recent epidemiological evidence suggests that ingestion of flavan 3-ols monomers reduces the risk of coronary heart disease.(12,13) These reports showed a strong inverse association between intake of (+)-catechin and (−)-epicatechin as flavan 3-ols monomers and death from coronary heart disease. Epidemiological evidence also suggests that ingestion of chocolate reduces the risk of cardiovascular diseases such as stroke and cardiometabolic diseases.(14,15) Buitrago-Lopez et al.(14) using six cohort studies and one cross-sectional study showed that the highest level of chocolate consumption was associated with a 37% reduction in cardiovascular disease (relative risk 0.63, 95% CI 0.44–0.90) and a 29% reduction in stroke compared with the lowest levels (Fig. 2). Larsson et al.(15) also reported a meta-analysis of 5 studies that showed the multivariable relative risk of stroke was 0.83 (95% CI 0.70–0.99) for the highest quartile of chocolate consumption (median 62.9 g/week) compared with the lowest quartile (median 0 g/week). Based on observational evidence, these results suggested that the level of chocolate consumption was associated with a substantial reduction in the risk of cardiovascular disorders.
Fig. 2.
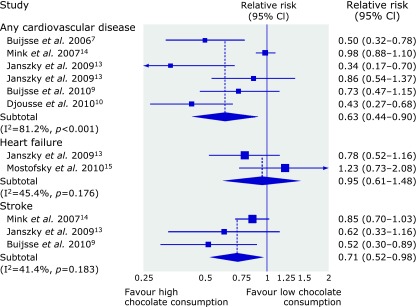
Relative risks for cardiovascular disease, heart failure, and stroke in adults with higher levels of chocolate consumption compared with lower levels. Reproduced from (14) with permission.
Association of Ingestion of Chocolate and Metabolic Syndrome Risk Factors
Numerous randomized, controlled trials (RCT) have investigated the effects of chocolate or cocoa products, especially dark chocolate, on metabolic syndrome risk factors such as hypertension,(16,17) vascular endothelial dysfunction,(18,19) dyslipidemia,(20,21) and glucose intolerance.(22,23) As shown in Table 1, seven meta-analyses of chocolate intervention trials(24–30) have been reported recently. Hooper et al.(30) analyzed the data of 1297 subjects in 42 acute or short-term chronic RCTs and showed that insulin resistance (HOMA-IR: −0.67; 95% CI: −0.98, −0.36) was improved by consumption of chocolate or cocoa due to significant reductions in serum insulin. They also reported that flow-mediated dilatation (FMD) improved after chronic (1.34%; 95% CI: 1.00%, 1.68%) and acute (3.19%; 95% CI: 2.04%, 4.33%) chocolate ingestion. Reductions in diastolic blood pressure (BP; −1.60 mmHg; 95% CI: −2.77, −0.43 mmHg) and mean arterial pressure (−1.64 mmHg; 95% CI: −3.27, −0.01 mmHg), and marginally significant improvements in LDL (−0.07 mmol/l; 95% CI: −0.13, 0.00 mmol/l) and HDL cholesterol levels (0.03 mmol/L; 95% CI: 0.00, 0.06 mmol/l) were also observed in the study. These data are consistent with the beneficial effects of cocoa products on metabolic syndrome risk factors shown in short-term intervention trials. However, further larger and longer-duration trials are required to confirm the potential cardiovascular benefits of cocoa flavan-3-ols.
Table 1.
Effect of chocolate on cardiovascular health: systematic reviews and meta analyses
| n (study) | n (subjects) | ||||
|---|---|---|---|---|---|
| Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A.(30) | Am J Clin Nutr 2012; 95: 740–751. | Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials | 42 | 1297 | HOMA-IR: −0.67; 95% CI: −0.98, −0.36 chronic FMD: 1.34%; 95% CI: 1.00%, 1.68% acuteFMD: 3.19%; 95% CI: 2.04%, 4.33% MBP: −1.64 mmHg; 95% CI: −3.27, −0.01 mmHg LDL: −0.07 mmol/l; 95% CI: −0.13, 0.00 mmol/l HDL: 0.03 mmol/l; 95% CI: 0.00, 0.06 mmol/l |
| Shrime MG, Bauer SR, McDonald AC, Chowdhury NH, Coltart CE, Ding EL.(29) | J Nutr 2011; 141: 1982–1988. | Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies | 24 | 1106 | SBP: −1.63 mmHg (p = 0.033) LDL: −0.077 mmol/l (p = 0.038) HDL: 0.046 mmol/l (p = 0.037) HOMA-IR: −0.94 points (p<0.001) FMD: 1.53% (p<0.001) |
| Tokede OA, Gaziano JM, Djoussé L.(28) | Eur J Clin Nutr 2011; 65: 879–886. | Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis | 10 | 320 | TC: −6.23 mg/dl (−11.60, −0.85 mg/dl) LDL: −5.90 mg/dl (−10.47, −1.32 mg/dl) HDL: −0.76 mg/dl (−3.02 – 1.51 mg/dl) TG: −5.06 mg/dl (−13.45 – 3.32 mg/dl) |
| Ried K, Sullivan T, Fakler P, Frank OR, Stocks NP.(27) | BMC Med 2010 Jun 28; 8: 39. | Does chocolate reduce blood pressure? A meta-analysis | 13 | 288 | SBP: −3.2 +/− 1.9 mmHg (p = 0.001) DBP: −2.0 +/− 1.3 mmHg (p = 0.003) |
| Jia L, Liu X, Bai YY, Li SH, Sun K, He C, Hui R.(13) | Am J Clin Nutr 2010; 92: 218–225. | Short-term effect of cocoa product consumption on lipid profile: a meta-analysis of randomized controlled trials | 8 | 215 | TC: 5.82 mg/dl (95% CI: −12.39, 0.76; p = 0.08) LDL: −5.87 mg/dl (95% CI: −11.13, −0.61; p<0.05) |
| Desch S, Schmidt J, Kobler D, Sonnabend M, Eitel I, Sareban M, Rahimi K, Schuler G, Thiele H.(26) | Am J Hypertens 2010; 23: 97–103. | Effect of cocoa products on blood pressure: systematic review and meta-analysis | 10 | 297 | MBP: −4.5 mmHg (−5.9 – −3.2, p<0.001) SBP: −2.5 mmHg (−3.9 – −1.2, p<0.001) |
| Taubert D, Roesen R, Schömig E(25) | Arch Intern Med 2007; 167: 626–634. | Effect of cocoa and tea intake on blood pressure: a meta-analysis | 5 | 173 | MBP: −4.7 mmHg (−7.6 – −1.8 mmHg; p = 0.002) SBP: −2.8 mmHg (−4.8 – −0.8 mmHg; p = 0.006) |
Bioavailability of Flavan 3-ol
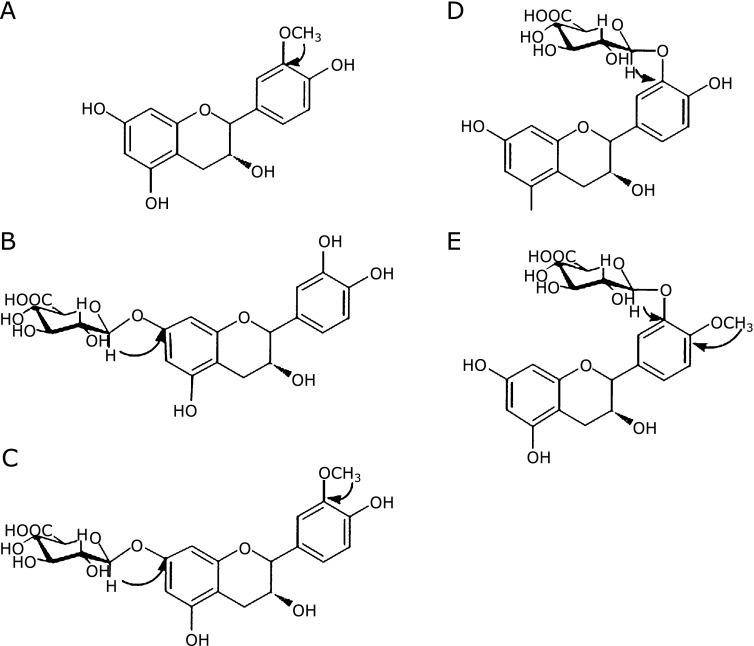
Numerous reports have investigated the bioavailability of flavan 3-ols. Flavan 3-ols monomers, such as (−)-epicatechin and (+)-catechin are well absorbed, and are metabolized mainly in the small intestine or liver, forming sulfate, glucuronide or methylated metabolites through the action of sulfotransferases (SULT), uridine-5'-diphosphate glucuronosyltransferases (UGTs) and catechol-O-methyltransferases (COMT),(31) respectively. Non-metabolized flavan 3-ol monomers are therefore rarely detected in the blood. We provided evidence that the chemical structure of (−)-epicatechin glucuronide, a major metabolite of (−)-epicatechin, was different between human and rats(Fig. 3).(32) The antioxidative activities of those metabolites was also shown to be reduced in metabolites derived from human biomaterials.(33)
Fig. 3.
Structure of (–)-epicatechin metabolites. (A) 3'-O-Methyl-(–)-epicatechin, (B) (–)-epicatechin-7-O-glucuronide, (C) 3'-O-Methyl-(–)-epicatechin-7-O-glucuronide, (D) (–)-epicatechin-3'-O-glucuronide, (E) 4'-O-Methyl-(–)-epicatechin-3'-O-glucuronide. Chemicals A, B and C were obtained from rat urine, D and E were obtained from human.
In contrast, there are numerous feeding studies on animals and humans that demonstrate polymeric epicatechin as procyanidins are not absorbed.(34) For example, we showed that only about 0.5% of the epicatechin dimer, procyanidin B2, is absorbed,(35) with the majority passing unaltered into the large intestine where it is catabolized by colonic microflora to a diverse range of phenolic acids(36,37) including 3-(3-hydroxyphenyl)propionic acid and 4-O-methylgallic acid.(38) These acids are then absorbed into the circulatory system and excreted in the urine. It is possible the biological effects of procyanidins described above are attributable to these phenolic acids, although there is a lack of detailed information in this area.
Flavan 3-ols Bioactivity: In Vitro Studies
As described above, despite the bioavailability of flavan 3-ols being very low, there has been a large number of in vitro studies to examine improvements in metabolic syndrome risk factors following the ingestion of these compounds. Studies using cell culture or isolated organs showed that the nitric oxide (NO) radical, a potent endothelium dilatation factor, and endothelial nitric oxide synthase were increased by the addition of flavan 3-ols.(39–41) However, almost all these investigations lacked physiological significance as the parent compounds rather than the metabolites were used at high levels than those achieved in blood following oral administration of flavan 3-ols. Several recent studies have investigated flavan 3-ols-conjugated metabolites in mammals and microbial degradation products, with one study showing that O-methylated epicatechin inhibited NADPH oxidase in the endothelium.(42) Phenolic acids, which are metabolites of colonic fermentation, have also been reported to possess certain bioactivities.(43,44) Unfortunately, biological significance was also not achieved in these studies due to the high dose of metabolites used in the experiments. Taken together, these studies suggest that absorbed procyanidins, catechins or phenolic acids contributed only a portion of the improvement in metabolic syndrome risk factors.
Flavan 3-ols Bioactivities—a New Angle of Observation
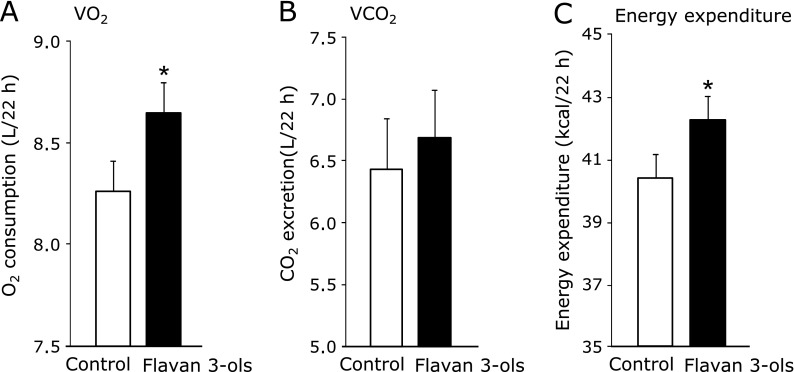
A study in over 1000 American men and women showed a negative correlation between the frequency of chocolate consumption and body mass index (BMI) (Table 2).(45) Taub et al.(46) also reported that ingestion of chocolate stimulated mitochondrial biogenesis of skeletal muscle in patients with type 2 diabetes or heart failure. We showed recently that repeated ingestion of the flavan 3-ols fraction influenced energy expenditure in rats.(47) In that study, the animals were fed for 2 weeks with either a normal diet or one containing 0.2% flavan 3-ols derived from cacao. At the end of the experimental period, energy expenditure was estimated by an indirect calorimetric method that measured oxygen consumption (VO2) and carbon dioxide excretion (VCO2) for 22 h. As shown in Fig. 4, total O2 consumption was increased significantly in the flavan 3-ols group compared with controls. As a consequence, total energy expenditure also increased significantly in the flavan 3-ols group. We observed that repeated ingestion of flavan 3-ols reduced mean blood pressure to the same degree as that reported in published meta-analyses. In contrast, a single administration of flavan 3-ols in rats was shown to cause an immediate elevation in blood pressure and heart rate leading to increased blood flow and recruitment of capillaries in skeletal muscle (Table 3)(48). In addition, studies by Yamashita et al.(49) demonstrated that flavan 3-ols prevented glucose intolerance and obesity by promoting translocation of glucose transporter 4 and phosphorylation of AMP-activated protein kinase (AMPK) in the plasma membrane of skeletal muscle and brown adipose tissues.
Table 2.
Chololate consumption frequency predicts lower BMI: regression results(45)
| Adjustment model | Chocolate consumption frequency, association with BMI | |
|---|---|---|
| δ (SE) | p value | |
| Unadjusted | –0.142 (0.053) | 0.08 |
| Age and sex adjusted | –0.126 (0.053) | 0.02 |
| Age, sex and activity adjusted | –0.130 (0.052) | 0.01 |
| Age, sex and calorie adjusted | –0.146 (0.059) | 0.01 |
| Age, sex and satfat adjusted | –0.190 (0.059) | 0.001 |
| Age, sex, satfat and CES-D adjusted | –0.191 (0.059) | 0.001 |
| Age, sex, satfat, fruite and vegetable, and CES-D adjusted | –0.201 (0.060) | 0.001 |
| Age, sex, satfat, fruite and vegetable, and CES-D and calories adjusted | –0.208 (0.060) | 0.001 |
Fig. 4.
VO2 (A), VCO2 (B) and energy expenditure (C) in rats fed control or 0.2% flavan 3-ols containing diet. Values are mean and SD. Significantly different from control, *p<0.05.
Table 3.
Influence of single oral administration of cocoa or flavan 3-ols on microcirculation in rat cremaster muscle
| n | ΔRBC velocity (µm/s) | Δnewly recruited capillary (number) | Δheart Rate (beats/min) | Δmean blood pressure (mmHg) | ||
|---|---|---|---|---|---|---|
| Vehicle | 8 | 5 min | 1.2 ± 2.6 | 4.3 ± 0.8 | –2.1 ± 1.8 | –4.6 ± 4.3 |
| 20 min | –0.6 ± 3.1 | 8.6 ± 3.1 | 3.2 ± 8.2 | 2.1 ± 3.6 | ||
| Cocoa | 8 | 5 min | 61.2 ± 23.3** | 8.2 ± 1.3 | 7.3 ± 3.6 | 14.2 ± 9.6 |
| 20 min | 116 ± 26.2** | 19.6 ± 5.6** | 12.2 ± 4.2* | 30.2 ± 7.8* | ||
| Flavan 3-ols | 8 | 5 min | 58.4 ± 29.7** | 7.6 ± 1.4 | 6.3 ± 2.8 | 13.8 ± 8.3 |
| 8 | 20 min | 98.6 ± 35.6** | 19.1 ± 4.2** | 14.6 ± 3.1* | 28.9 ± 8.8* |
Each value represnts the mean ± SD. Significantly difference from vehicle; *p<0.05, **p<0.01.
These results in recent reports are summarized in Fig. 5. A hypotensive effect is produced by the oral administration of flavan 3-ols that induced expression of endothelial nitrogen oxide synthase (eNOS), while this effect is unclear in a point whether this effect was produced by metabolites of monomers in circulating blood or oligomers that remained in the gastrointestinal tract. In skeletal muscle, enhancement of energy expenditure is induced by oral administration of flavan 3-ols following with AMPK activation. AMPK activation enhanced both transcription and translocation of glucose transporter type 4 (GLUT4), resulting in acceleration of glucose uptake. It has been shown that AMPK also activates peroxisome-proliferator-activated receptor coactivator 1(PGC1α) which is a key factor in mitochondrial biogenesis. Improvement of dyslipidemia or lowering of BMI in RCT or epidemiological studies may also be induced by this mitochondrial biogenesis promoting effect. On the basis of these results, recent studies have attempted to define the mechanism responsible for the beneficial effects of flavan 3-ols from a new perspective.
Fig. 5.
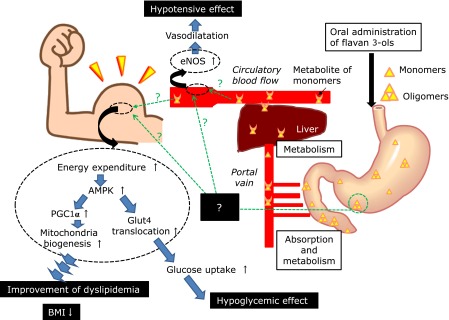
The results indicated these recent reports were summarized as Table 5. Hypotensive effect was shown by oral administrated flavan 3-ols through induced endothelial nitrogen oxide synthase (eNOS) expression. In skeletal muscle, enhancement of energy expenditure was induced by oral administration of flavan 3-ols, it resulted activation of AMPK. AMPK activation enhanced both transcription and translocation of glucose transporter type 4 (GLUT4), resulting acceleration of glucose uptake. AMPK might be activated peroxisome-proliferator- activated receptor coactivator 1 (PGC1α) which was the key factor of mitochondrial biogenesis. Improvement of dyslipidemia or BMI lowering activity seen in RCT or epidemiological studies also might be induced by such mitochondria biogenesis promoting effect.
Conclusion
In conclusion, flavan 3-ols may improve hypertension, dyslipidemia, insulin resistance, and obesity induced by inappropriate daily habits. However, further studies are required to elucidate the mechanisms responsible for the risk reduction of cardiovascular diseases caused by flavan 3-ols.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hammerstone JF, Lazarus SA, Mitchell A, Rucker R, Schmitz HH. Identification of flavan 3-ol s in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography/mass spectrometry. J Agric Food Chem. 1999;47:490–496. doi: 10.1021/jf980760h. [DOI] [PubMed] [Google Scholar]
- 2.Hatano T, Miyatake H, Natsume M, et al. Proanthocyanidin glycosides and related polyphenols from cacao liquor and their antioxidant effects. Phytochemistry. 2002;59:749–758. doi: 10.1016/s0031-9422(02)00051-1. [DOI] [PubMed] [Google Scholar]
- 3.Sanbongi C, Osakabe N, Natsume M, Takizawa T, Gomi S, Osawa T. Antioxidative polyphenols isolated from theobroma cacao. J Agric Food Chem. 1998;46:454–457. doi: 10.1021/jf970575o. [DOI] [PubMed] [Google Scholar]
- 4.Mao TK, Van De Water J, Keen CL, Schmitz HH, Gershwin ME. Cocoa flavonols and procyanidins promote transforming growth factor-β1 homeostasis in peripheral blood mononuclear cells. Exp Biol Med. 2003;228:93–99. doi: 10.1177/153537020322800113. [DOI] [PubMed] [Google Scholar]
- 5.Keen CL, Holt RR, Oteiza PI, Fraga CG, Schmitz HH. Cocoa antioxidants and cardiovascular health. Am J Clin Nutr. 2005;81 (1 Suppl):298S–303S. doi: 10.1093/ajcn/81.1.298S. [DOI] [PubMed] [Google Scholar]
- 6.Aron PM, Kennedy JA. Flavan-3-ols: nature, occurrence and biological activity. Mol Nutr Food Res. 2008;52:79–104. doi: 10.1002/mnfr.200700137. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Micaelo N, González-Abuín N, Ardèvol A, Pinent M, Blay MT. Procyanidins and inflammation: molecular targets and health implications. Biofactor. 2012;38:257–265. doi: 10.1002/biof.1019. [DOI] [PubMed] [Google Scholar]
- 8.Selmi C, Mao TK, Keen CL, Schmitz HH, Eric Gershwin M. The anti-inflammatory properties of cocoa flavanols. J Cardiovasc Pharmacol. 2006;47 (Suppl 2):S163–S171. doi: 10.1097/00005344-200606001-00010. [DOI] [PubMed] [Google Scholar]
- 9.De Curtis A, Amore C, Donati MB, De Gaetano G, Iacoviello L. A procyanidin extract prolongs bleeding time but does not prevent thrombosis in rats. J Thromb Haemost. 2003;1:199–200. doi: 10.1046/j.1538-7836.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 10.Corti R, Flammer AJ, Hollenberg NK, Lüscher TF. Cocoa and cardiovascular health. Circulation. 2009;119:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 11.McCullough ML, Chevaux K, Jackson L, et al. Hypertension, the Kuna, and the epidemiology of flavonoids. J Cardiovasc Pharmacol. 2006;47:S103–S109. doi: 10.1097/00005344-200606001-00003. [DOI] [PubMed] [Google Scholar]
- 12.Arts IC, Hollman PC, Feskens EJ, Bueno de Mesquita HB, Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am J Clin Nutr. 2001;74:227–232. doi: 10.1093/ajcn/74.2.227. [DOI] [PubMed] [Google Scholar]
- 13.Arts IC, Jacobs DR, Jr, Harnack LJ, Gross M, Folsom AR. Dietary catechins in relation to coronary heart disease death among postmenopausal women. Epidemiology. 2001;12:668–675. doi: 10.1097/00001648-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Buitrago-Lopez A, Sanderson J, Johnson L, et al. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011;26:d4488. doi: 10.1136/bmj.d4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsson SC, Virtamo J, Wolk A. Chocolate consumption and risk of stroke: a prospective cohort of men and meta-analysis. Neurology. 2012;79:1223–1229. doi: 10.1212/WNL.0b013e31826aacfa. [DOI] [PubMed] [Google Scholar]
- 16.Taubert D, Roesen R, Lehmann C, Jung N, Schömig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49–60. doi: 10.1001/jama.298.1.49. [DOI] [PubMed] [Google Scholar]
- 17.Desch S, Kobler D, Schmidt J, et al. Low vs. higher-dose dark chocolate and blood pressure in cardiovascular high-risk patients. Am J Hypertens. 2010;23:694–700. doi: 10.1038/ajh.2010.29. [DOI] [PubMed] [Google Scholar]
- 18.Engler MB, Engler MM, Chen CY, et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J Am Coll Nutr. 2004;23:197–204. doi: 10.1080/07315724.2004.10719361. [DOI] [PubMed] [Google Scholar]
- 19.Schroeter H, Heiss C, Balzer J, et al. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baba S, Osakabe N, Kato Y, et al. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am J Clin Nutr. 2007;85:709–717. doi: 10.1093/ajcn/85.3.709. [DOI] [PubMed] [Google Scholar]
- 21.Baba S, Natsume M, Yasuda A, et al. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J Nutr. 2007;137:1436–1441. doi: 10.1093/jn/137.6.1436. [DOI] [PubMed] [Google Scholar]
- 22.Grassi D, Necozione S, Lippi C, et al. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46:398–405. doi: 10.1161/01.HYP.0000174990.46027.70. [DOI] [PubMed] [Google Scholar]
- 23.Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- 24.Taubert D, Roesen R, Schömig E. Effect of cocoa and tea intake on blood pressure: a meta-analysis. Arch Intern Med. 2007;167:626–634. doi: 10.1001/archinte.167.7.626. [DOI] [PubMed] [Google Scholar]
- 25.Hooper L, Kroon PA, Rimm EB, et al. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 26.Desch S, Schmidt J, Kobler D, et al. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens. 2010;23:97–103. doi: 10.1038/ajh.2009.213. [DOI] [PubMed] [Google Scholar]
- 27.Ried K, Sullivan T, Fakler P, Frank OR, Stocks NP. Does chocolate reduce blood pressure? A meta-analysis. BMC Med. 2010;8:39. doi: 10.1186/1741-7015-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tokede OA, Gaziano JM, Djoussé L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr. 2011;65:879–886. doi: 10.1038/ejcn.2011.64. [DOI] [PubMed] [Google Scholar]
- 29.Shrime MG, Bauer SR, McDonald AC, Chowdhury NH, Coltart CE, Ding EL. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. J Nutr. 2011;141:1982–1988. doi: 10.3945/jn.111.145482. [DOI] [PubMed] [Google Scholar]
- 30.Hooper L, Kay C, Abdelhamid A, et al. Effects of chocolate, cocoa, and flavan 3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95:740–751. doi: 10.3945/ajcn.111.023457. [DOI] [PubMed] [Google Scholar]
- 31.Donovan JL, Manach C, Faulks RM, Kroon PA. Absorption and metabolism of plant secondary metabolites. In: Crozier A, Clifford MN, Ashihara H, editors. Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet. Oxford: Blackwell Publishing; 2006. pp. 303–351. [Google Scholar]
- 32.Natsume M, Osakabe N, Oyama M, et al. Structures of (–)-epicatechin glucuronide identified from plasma and urine after oral ingestion of (–)-epicatechin: differences between human and rat. Free Radic Biol Med. 2003;34:840–849. doi: 10.1016/s0891-5849(02)01434-x. [DOI] [PubMed] [Google Scholar]
- 33.Natsume M, Osakabe N, Yasuda A, et al. In vitro antioxidative activity of (–)-epicatechin glucuronide metabolites present in human and rat plasma. Free Radic Res. 2004;38:1341–1348. doi: 10.1080/10715760400022087. [DOI] [PubMed] [Google Scholar]
- 34.Espín JC, García-Conesa MT, Tomás-Barberán FA. Nutraceuticals: facts and fiction. Phytochemistry. 2007;68:2986–3008. doi: 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Baba S, Osakabe N, Natsume M, Terao J. Absorption and urinary excretion of procyanidin B2 [epicatechin-(4β-8)-epicatechin] in rats. Free Radic Biol Med. 2002;33:142–148. doi: 10.1016/s0891-5849(02)00871-7. [DOI] [PubMed] [Google Scholar]
- 36.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81 (1 Suppl):230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 37.Appeldoorn MM, Vincken JP, Aura AM, Hollman PC, Gruppen H. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-γ-valerolactone as the major metabolites. J Agric Food Chem. 2009;57:1084–1092. doi: 10.1021/jf803059z. [DOI] [PubMed] [Google Scholar]
- 38.Ward NC, Croft KD, Puddey IB, Hodgson JM. Supplementation with grape seed polyphenols results in increased urinary excretion of 3-hydroxyphenylpropionic Acid, an important metabolite of proanthocyanidins in humans. J Agric Food Chem. 2004;52:5545–5549. doi: 10.1021/jf049404r. [DOI] [PubMed] [Google Scholar]
- 39.Matsui T, Korematsu S, Byun EB, Nishizuka T, Ohshima S, Kanda T. Apple procyanidins induced vascular relaxation in isolated rat aorta through NO/cGMP pathway in combination with hyperpolarization by multiple K+ channel activations. Biosci Biotechnol Biochem. 2009;73:2246–2251. doi: 10.1271/bbb.90334. [DOI] [PubMed] [Google Scholar]
- 40.Fitzpatrick DF, Bing B, Maggi DA, Fleming RC, O’Malley RM. Vasodilating procyanidins derived from grape seeds. Ann N Y Acad Sci. 2002;957:78–89. doi: 10.1111/j.1749-6632.2002.tb02907.x. [DOI] [PubMed] [Google Scholar]
- 41.Tokoudagba JM, Auger C, Bréant L, et al. Procyanidin-rich fractions from Parkia biglobosa (Mimosaceae) leaves cause redox-sensitive endothelium-dependent relaxation involving NO and EDHF in porcine coronary artery. J Ethnopharmacol. 2010;132:246–250. doi: 10.1016/j.jep.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 42.Steffen Y, Gruber C, Schewe T, Sies H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch Biochem Biophys. 2008;469:209–219. doi: 10.1016/j.abb.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Sanae F, Miyaichi Y, Hayashi H. Potentiation of vasoconstrictor response and inhibition of endothelium-dependent vasorelaxation by gallic acid in rat aorta. Planta Med. 2002;68:690–693. doi: 10.1055/s-2002-33806. [DOI] [PubMed] [Google Scholar]
- 44.Na HJ, Lee G, Oh HY, et al. 4-O-Methylgallic acid suppresses inflammation-associated gene expression by inhibition of redox-based NF-κB activation. Int Immunopharmacol. 2006;6:1597–1608. doi: 10.1016/j.intimp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Golomb BA, Koperski S, White HL. Association between more frequent chocolate consumption and lower body mass index. Arch Intern Med. 2012;172:519–521. doi: 10.1001/archinternmed.2011.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taub PR, Ramirez-Sanchez I, Ciaraldi TP, et al. Alterations in skeletal muscle indicators of mitochondrial structure and biogenesis in patients with type 2 diabetes and heart failure: effects of epicatechin rich cocoa. Clin Transl Sci. 2012;5:43–47. doi: 10.1111/j.1752-8062.2011.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osakabe N. Cacao polyphenol. In: Hatano T, editor. Polyphenols Functional Constituents of Medicinal Plants and Foods. CMC Publishing CO. Ltd; 2012. pp. 231–247. [Google Scholar]
- 48.Osakabe N. Polyphenols in Theobroma cacao ameliorate microcirculation: in vivo intravital microscopic observation in rats. J Food Drug Anal. 2012;20 (Suppl 1):288–291. [Google Scholar]
- 49.Yamashita Y, Okabe M, Natsume M, Ashida H. Prevention mechanisms of glucose intolerance and obesity by cacao liquor procyanidin extract in high-fat diet-fed C57BL/6 mice. Arch Biochem Biophys. 2012;527:95–104. doi: 10.1016/j.abb.2012.03.018. [DOI] [PubMed] [Google Scholar]