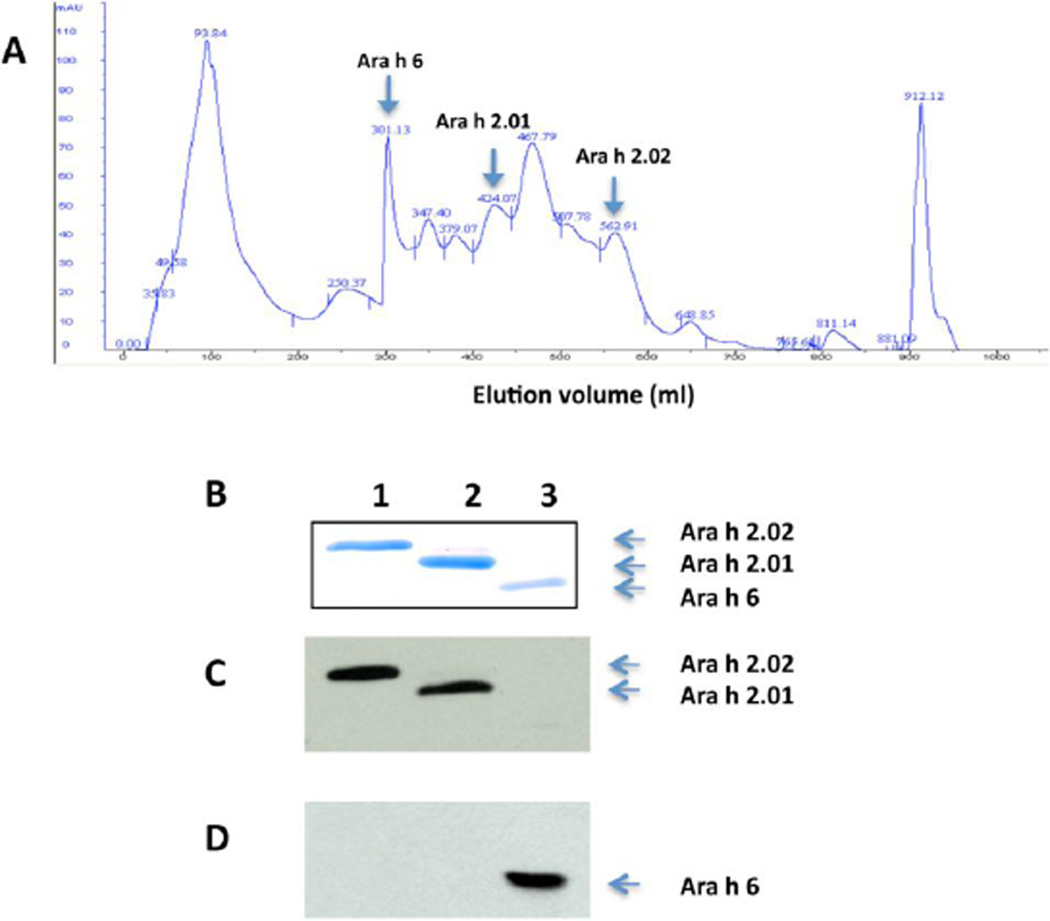
Figure 2. Purification of native Ara h 2.01, Ara h 2.02 and Ara h 6 from the 20kD fraction by hydrophobic interaction chromatography (HIC).
A) Absorption at 280 nm is shown on the vertical axis (mAU). Highly purified Ara h 2.01, 2.02 and Ara h 6 fractions were found in fractions at 305–340 ml, 428–435 ml and 570–580 ml respectively as noted. B) Coomassie blue staining of an SDS-PAGE gel:lane 1, Ara h 2.02(~19 kD); Lane 2, Ara h 2.01 (~ 17 kD; and Lane 3, Ara h 6 (~ 15 kD). C) Immunoblot of purified Ara h 2 and 6 with rabbit anti-Ara h 2 antibody, and D) Immunoblot of purified Ara h 2 and 6 with rabbit anti- Ara h 6 antibody. This purification scheme has been repeated more than 5 times with similar results.