Abstract
Prior work by our group has shown the feasibility, safety, and validity of a multi-day, multi-dose paradigm of self-regulated cocaine administration in humans. The current work sought to consolidate these methods in a single-day design focused on reducing logistical complexity, decreasing research burden to human subjects, and increasing suitability for medication development designs. Methods: Eleven experienced cocaine users participated in a 6-hour, single-day design, consisting of one safety/eligibility and three experimental cocaine periods (during which subjects were allowed to self-administer 8, 16, and 32 mg/70 kg cocaine doses under a fixed-ratio 1: 5 minute timeout schedule). Changes in cocaine-induced cardiovascular response, self-administration behavior, and subjective effects were assessed. Results: Procedures were well tolerated by participants, and no significant adverse events were noted. Significant (p<0.05), changes in measures of cocaine self-administration (e.g., responses, infusions, interinfusion intervals, consumption, and plasma levels), cardiovascular response (HR), and subjective effects (“high”) were observed. In contrast, cocaine-induced increases in other vital signs (e.g., SBP, DBP) and subjective effect measures (e.g., paranoia) did not differ between doses. Conclusions: These data support the safety, tolerability and validity of our single-day design. Depending on the application, such methods may afford advantages for assessing the self-regulation of cocaine administration behavior in humans (e.g., including medication development designs).
Keywords: cocaine self-administration, human studies, self-regulation, cocaine plasma levels, subjective effects
Introduction
Human laboratory studies involving cocaine administration have contributed significantly to our understanding of the drug’s pharmacology, including cardiovascular effects (Fischman et al. 1976; Foltin et al. 1995), drug tolerance (Foltin and Fischman 1991a), subjective states (Lynch et al 2006; Sughondhabirom et al. 2005; Foltin et al. 2003; Fischman and Foltin 1992b; Fischman 1989), abuse liability (Fischman et al. 1983a,b; Foltin and Fischman 1991c), regulation of drug intake (Angarita et al. 2010), effects on neuropsychological functioning (e.g., sleep and cognition) (Matuskey et al 2010; Morgan and Malison, 2008; Morgan et al., 2006; Pace-Schott et al., 2008), and in conjunction with neuroimaging, brain mechanisms (Martinez et al 2004, 2007; Risinger et al 2005; Schlaepfer et al 1997; Breiter et al 1997). Moreover, human laboratory studies have played an increasingly important role in the development of improved treatments for cocaine dependence, including investigations of preliminary safety (e.g., potential drug-drug interactions) and efficacy (Penetar et al 2006; Collins et al 2001; Oliveto et al 2001; Sofuoglu et al 2005; Grasing et al 2009; Hart et al 2006, 2008; Rotheram-Fuller et al 2006; Rush et al 2010; Walsh et al 2001; Evans et al. 2001; Newton et al 1999; Fischman et al. 1990; Haberny et al. 1995; Haney et al. 1998, 1999, 2001, Kalayasiri et al., 2007a).
Our group has developed a multi-day paradigm of “self-regulated” (i.e., ad libitum) cocaine administration (Sughondhabirom et al 2005; Lynch et al 2005) in which experienced users are allowed flexibility in the control of the frequency of cocaine intake. Current procedures require several daily sessions of self-administration (e.g. 2 hour ‘binge’ sessions on successive days) to allow for the testing of multiple cocaine doses (e.g., 8-32 mg/70kg IV). Using four and five day designs, we have shown these methods to be safe and well-tolerated, pharmacologically valid (i.e., dose responsive with respect to cardiovascular, behavioral and subjective effects) and test-retest reliable (Sughondhabirom et al 2005; Lynch et al 2005; Kalayasiri et al 2006). We have previously used these methods to study the effects of candidate pharmacotherapies (e.g., disulfiram; Kalayasiri et al 2007a), gender (Lynch et al. 2008) and genetics (Kalayasiri et al 2007b) on cocaine-related effects, as well as potential differences in the self-regulation of drug administration by humans (Angarita et al. 2010).
While advantages of reliability, validity, and full-dose response remain, our procedures, as currently implemented, are time-intensive and require participants to undergo multiple test sessions spread over several (e.g., 4-5) days. The latter has also provided challenges of logistics and/or feasibility for medications development (e.g., due to research burden placed upon human subjects and institutional/facility resources), with dose-finding designs typically employing multiple doses of the drug candidate (e.g., a placebo-controlled study of two active medications and three active cocaine doses would require 12-16 laboratory sessions, as currently implemented). Therefore, in the current study, we assessed whether salient features of our methods (e.g., self-regulation and full cocaine dose-response curves) could be preserved in the context of a more practical, single-day design, maintaining essential elements of safety, subject tolerability, and pharmacologic validity.
Methods
Subject population
Participants were medically healthy, non-treatment seeking cocaine-dependent individuals who were recruited through local newspaper advertisements and by word-of-mouth referrals. Initial screening evaluations were typically conducted on an outpatient basis and individuals underwent an unstructured psychiatric interview, physical and neurological examinations, ECG, and routine laboratory testing (i.e., blood chemistries, hematology, and urinalysis). Participants were required to meet DSM-IV criteria for cocaine abuse or dependence, be between the ages of 18 and 50 years, report cocaine use via smoked and/or IV routes of administration (although all participants preferred smoked use and only one admitted to past IV cocaine use), have a history of regular, recent use greater than the maximum employed in the study (i.e. ≥728mg in a day), and provide objective evidence of recent use (i.e., benzoylecgonine positivity) on urine toxicology testing.
Exclusion criteria included nonsubstance related Axis I psychiatric disorders, cardiac conditions, seizures, diabetes, any current systemic medications, sedative hypnotic or opiate dependence, and for females, a positive serum β-HCG (i.e., pregnancy) test.
All participants provided voluntary written informed consent, and all study-related procedures were approved by both the Yale University Human Investigation Committee and the Yale Center for Clinical Investigation’s Safety and Science Committee. A certificate of confidentiality was obtained from the National Institute on Drug Abuse (NIDA), and the study was conducted under the auspices of an Investigational New Drug (IND) application to the U.S. Food and Drug Administration (RTM, physician sponsor). All study participants were compensated for their participation.
Of the 16 individuals that consented to the study, two were excluded after an abnormal screening ECG and two individuals did not complete the study: both passed the safety/eligibility assessments but one had sustained blood pressure elevations above safety criteria in response to repeated cocaine, and the other subject withdrew electively (stating he was not comfortable being observed while using cocaine). Additionally, one subject (completer) responded at very low levels on each of the test sessions (two or fewer responses) and was excluded on statistical grounds (> 3 standard deviations below mean self-administration rates).
General Study Design
Cocaine dependent subjects participated in a single, approximately 6-hour (5h and 50 min) cocaine self-administration study, comprised of an initial safety/eligibility phase (80 min) followed by a subsequent experimental component (4h and 30 min; see figure 1). The initial/safety component was comprised of three, serial (at 20 min intervals), bolus, fixed order, escalating dose injections of 8, 16, and 32 mg/70kg IV cocaine hydrochloride via patient-controlled analgesia (PCA) pump. Given the time constraints inherit in the current one-day design, in conjunction with our clear prior demonstration of differences in active vs. placebo response (Lynch et al 2006; Sughondhabirom et al 2005), we opted to exclude a placebo session in the current design (with the primary goal of establishing the capacity of the current procedures to reproduce pharmacological dose-response relationships without placebo).
Figure 1.
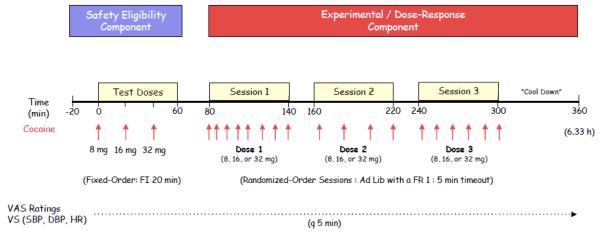
A schematic representing the cocaine self-administration day. See text for full description of the study design.
The purpose of the safety/eligibility phase was two-fold: 1) to familiarize subjects with the experimental methods (PCA pump, cocaine doses, experimental environment), and 2) to minimize chances of unsafe cardiovascular responses by establishing each subject’s ability to tolerate IV cocaine over the full range of doses tested in subsequent experimental components. Subjects who exhibited elevations in vital signs above safety thresholds and/or evidence of clinically significant cardiac ectopy, arrhythmias, or symptoms were excluded from further participation. A twenty-minute cocaine-free period followed the safety/eligibility component to allow vital signs and subjective effects to subside.
For eligible subjects (N=14), the experimental component consisted of three “ad lib” one-hour self-administration periods (one dose per period) conducted in a randomized, double-blind fashion. Twenty-minute between-dose rest (i.e., cocaine-free) periods allowed for behavioral and cardiovascular effects to subside and for the medical personnel to change cocaine doses and draw cocaine blood levels. Subjects who exhibited increased vital signs above established safety thresholds had the PCA pump withheld until they returned below holding parameters.
All cocaine self-administration procedures were conducted with individuals without food or drink (NPO) at least five hours before testing. Sessions took place in a private room at the Yale Center for Clinical Investigation’s Hospital Research Unit (YCCI-HRU) at Yale-New Haven Hospital. Individuals were resting in a comfortable chair throughout the session with a touch screen laptop in front of them for ratings on cocaine use-related symptoms (e.g. paranoia, hunger, etc.). Participants had two IV lines placed in opposite antecubital or forearm veins for infusing cocaine and drawing plasma cocaine levels, respectively. Clock times of the PCA pump, cardiac monitor, and laptop were synchronized to ensure the temporal correspondence of independent measures. An advanced cardiac life support (ACLS) certified research nurse and a basic life support (BLS) certified research assistant were present during all cocaine sessions. Sessions were started in the morning (typically by 9:00–10:00 A.M.) and completed by early afternoon (typically by 3:00–4:00 P.M.). Subjects were monitored for a 30-min period after completing the cocaine self-administration component at which point the experiment was concluded and the subjects were escorted back to the Clinical Neuroscience Research Unit (CNRU).
Facility
Participants were admitted to a 12-bed inpatient psychiatric research facility (CNRU). The unit includes a structured daily routine and 24-hour nursing and medical staff coverage for research participants. Individuals resided on the unit for the duration of the study and participated in all activities (except for substance abuse treatment) when not actively engaged in the cocaine self-administration session. Meals and snacks were caffeine-free and provided four times per day. Per routine unit policies and procedures, all visitors and packages were screened and approved prior to being allowed on the unit. Urine toxicology assays were conducted three times per week and were negative with the exception of positive tests for cocaine consistent with laboratory cocaine administration. Fifteen minute ‘fresh air’ breaks were scheduled to allow for tobacco smoking. Following their involvement in the study and without respect to completion of study procedures, participants were offered voluntary referral to one of several local agencies for outpatient treatment.
Materials
Equipment
We employed a PCA pump (Curlin 6000 model; B. Braun Medical Inc., Bethlehem, PA.), which was loaded and programmed by medically trained research personnel. Presses of the corded PCA button (i.e., responses) elicited an audible beep from the pump and a corresponding drug infusion. The PCA pump delivered cocaine as a bolus injection of 1 ml over a 30 s interval followed by a 5 min lockout period. The 5 min lockout period is a factory set safety feature of the PCA pump that is designed to prevent individuals from receiving a subsequent drug infusion until a period of time sufficient for them to experience the effects of the preceding injection has elapsed. Additional responses on the push button during the 5-min lockout produced similar audible beeps but did not lead to pump activation. Given this lockout period, it was possible to obtain up to a total of 12 cocaine infusions each hour. Participants were told of the 5-minute lock out period at screening and again at the start of the self-administration day.
Cocaine
Cocaine hydrochloride was purchased from Mallinckrodt (St. Louis, MO, USA) and prepared and verified in laboratory analysis as described in previous studies (Sughondhabirom et al 2005; Lynch et al 2006). The experimental use of cocaine was conducted under a physician-sponsored IND application (RTM) with the Food and Drug Administration (FDA) and under appropriate local (i.e., State of Connecticut, Department of Consumer Protection) and federal (i.e., U.S. Drug Enforcement Agency or DEA) controlled substance licenses.
Outcome Measures
Several outcomes were obtained during the study including measures of physiological response (i.e., cardiovascular vital signs), cocaine self-administration, subjective effects and plasma cocaine levels. Heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were automatically obtained at 5-min intervals using a cardiac monitor with a self-inflating blood pressure cuff and transducer, with values recorded for the duration of the 6-hour self-administration session. If vital signs exceeded the maximal allowed values (i.e., 75% of age-adjusted maximum HR, 170 mmHg SBP, or 100 mmHg DBP) then the response button was temporarily taken away from the participant and returned to the subject only after vital sign checks revealed a return to levels below these same parameters.
Cocaine self-administration data included responses (button pushes) and infusions (cocaine deliveries) and was continuously recorded by the PCA pump (including all responses made during the 5 min lockout period, during infusions, and if a maximum dose was obtained). These timed measures were internally stored and then electronically downloaded for subsequent analysis. Derivative measures, including interinfusion intervals (calculated as the time between two consecutive infusions, excluding intervals in which pump access was withheld due to vital sign elevations or bathroom breaks) and cocaine consumed (calculated as the number of infusions times the infusion dose), were also obtained.
After each self-administered period (but not sooner than 15 minutes after the last infusion) two (i.e., replicate) blood samples were obtained for the determination of plasma cocaine concentrations. Control levels were obtained at baseline immediately prior to cocaine self-administration. All blood samples were drawn from the arm contralateral to cocaine infusions to prevent cross-contamination. This sampling strategy was employed because of the “ad lib” nature of our cocaine self-administration paradigm (i.e., individuals pressed for cocaine at different times) and cocaine’s biexponential kinetics (i.e., reflecting biodistribution and elimination). Thus, end of dose blood samples in relationship to cumulative cocaine intake over each self-administration period provided an index of cumulative drug exposure for analyses. Blood samples were drawn in gray-stoppered, vacutainer tubes containing sufficient sodium fluoride to prevent degradation of cocaine by plasma pseudocholinesterase. Samples were immediately centrifuged, and the plasma separated and stored at −70° C until the time of analysis. Plasma cocaine levels were assayed as previously described (Jatlow and Nadim 1990).
Measures of 12 subjective effects were collected every 5 min by visual analog scale (VAS) ratings that were scored on a scale of 0 to 10; not at all (0) at one end (left) and most ever (10) at the other (right). Ten VAS measures were labeled as “I feel…” “high”, “stimulated”, “talkative”, “good”, “bad”, “anxious”, “paranoid”, “hungry”, “restless”, and “tongue-tied”, and two as “I want cocaine” and “I want nicotine”. Ratings were entered on a laptop computer with a touch screen and custom written software (implemented in Microsoft Access), which automatically prompted subjects for their response at 5-min intervals over the entire self-administration session.
Data analysis
Outcome measures were assessed for normality by visual (e.g., graphical) and statistical methods (e.g., Kolmogorov– Smirnov test). Reponses and interinfusion self-administration data, and plasma cocaine levels were approximately normal after log-transformation. The following VAS measure were highly skewed and non-normal, even after transformation: “stimulated”, “bad”, “anxious”, “paranoid”, “hungry”, “restless”, and “tongue-tied”. In the case of normally distributed data, linear mixed models were employed which included cocaine dose (8, 16, and 32 mg) and time as within-subjects factors. The interaction between dose and time was modeled and the best-fitting variance-covariance structure was selected based on information criteria. Models for cocaine administration (e.g., infusions, response) excluded time as a factor. In the case of non-normally distributed data, measures were analyzed, using the same factors above, by the nonparametric method for longitudinal data by Brunner (2002), where the data were first ranked, and then fitted using a mixed effects model with an unstructured variance-covariance matrix and p-values adjusted for ANOVA-type statistics (ATS). All available data were used in the mixed effects models described above. Secondary models for vital sign measures and VAS outcomes included comparison of average baseline (0-20 min) levels (no cocaine) versus average levels during each dose of cocaine administration (0-60 min). Data were analyzed using SAS, version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Demographics
A summary of demographic information on subjects analyzed (N=11) is presented in Table 1.
Table 1.
Subject Demographics
| Age | 41 ± 7 years |
|---|---|
| Race/ Ethnicity | 7 African American, 3 European American, 1 Hispanic |
| Gender | 8 Males, 3 Females |
| Frequency of cocaine use (last month) |
4 days per week |
| Amount of cocaine use (last month) |
$235 ± 188 (3-4 grams in current market prices) per day. 4 subjects used $400 or more per day. |
| Lifetime cocaine use | 22 ± 8 years |
| Initial age of first cocaine use | 19±4 years old |
| Alcohol use (per week) |
17 ± 15 drinks (higher use subjects (N=4) had 34 ± 8 drinks per week) |
| Cannabis use (per month) |
< 1 joint (5 subjects denied cannabis use) |
| Abstinence since last cocaine use (days) |
11 ± 7 days |
| Education | 11 completed high school (HS) 2 with further post-HS education |
Mean ± SD
Vital signs
Self-administration data showed an interaction between cocaine doses and time (F22,346=2.1; p=<0.003) on HR, where HR increased significantly over time during 32mg cocaine administration. No main effects of, or interactions between, cocaine doses and time were observed for SBP or DBP. Changes in HR (F3,10=22.1; p=<0.0001), SBP (F3,30=21.8; p=<0.0001) and DBP (F3,30=8.25; p=<0.001) were all elevated above baseline following cocaine.
Three of eleven subjects had asymptomatic elevations in SBP that resulted in a temporary suspension of cocaine self-administration. Elevations occurred during 4 of 33 total dose sessions (N=1 at 8mg, 1 at 32mg dose, 2 at 16mg) or for 29 (1.5%) of the 1,980 total self-administration minutes (these vital sign elevations, along with bathroom breaks, resulted in 6 responses, infusions and inter-infusion intervals that were omitted from further analysis). No serious or clinically significant adverse medical or cardiac events were experienced by any subject.
Cocaine self-administration
Mean ± SD response rates (i.e. button presses) for cocaine differed significantly according to cocaine doses, or 30 ± 27 (range: 4-85) responses for 8mg, 18 ± 15 (5-55) for 16mg, and 10 ± 6 (3-22) for 32mg (main effect of cocaine dose: F2,20=4.97, p=0.02). Post-hoc testing significantly distinguished 8 and 32 mg (F1,20=9.84; p=0.005), 16 and 32 mg doses (F1,20=5.17; p=0.03), and showed a statistical trend for 8 and 16 mg doses (F1,20=3.21; p=0.09).
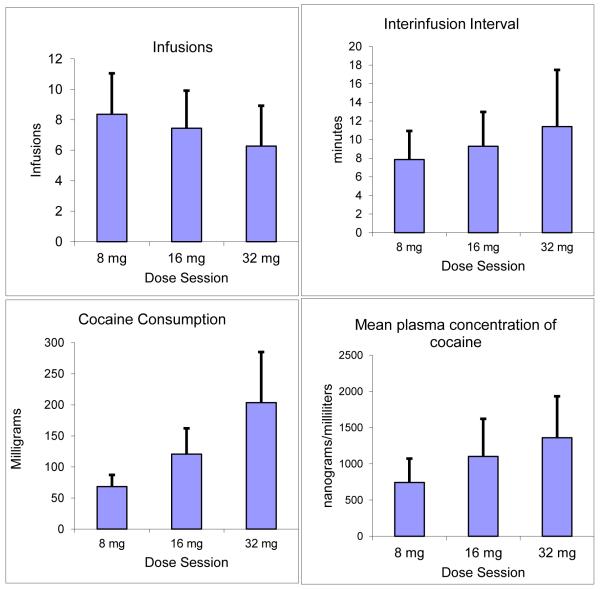
Analyses of infusion data similarly showed significant effects of cocaine doses (and less variable range) in fig. 1A (F2,20=4.4; p=0.03). Post hoc testing revealed significant differences in infusions for the 8 vs. 32mg doses (F1,20=8.83; p=<0.01).
Interinfusion interval data are depicted in Fig. 2B. Consistent with raw infusion data, interinfusion intervals showed a dose response relationship (i.e., increasing interval time with increasing bolus cocaine dose). Analysis of interinfusion intervals yielded a statistically significant main effect of cocaine dose (F2,20= 7.36; p=0.004) with post hoc testing confirming statistically meaningful trends or differences for comparisons of 8 vs. 16 mg (F1,20= 3.4; p=0.08), 8 vs. 32 mg (F1,20= 14.7; p=0.001), and 16 vs. 32 mg (F1,20= 3.97; p=0.06).
Figure 2.
Multi-dose (8, 16, and 32 mg) cocaine self-administration data (mean ± SD) are depicted, including cocaine infusions (2A), inter-infusion intervals (2B, cocaine consumed (in mg; 2C), and plasma cocaine levels (in ng/ml; 2D). Significant main effects of cocaine dose were observed for each outcome (p=0.0001 – 0.03; summarized in detail in the text). Significant between-dose, post-hoc-comparisons are also noted.
Similarly, cocaine consumption per period also varied as a function of dose (Fig. 1C). Dose-dependent effects were significant overall (main effect: F2,20=24.1; p=<0.0001) as well as between individuals doses (8 vs.16mg: F1,20=27, p=<0.0001; 8 vs. 32mg: F1,20=40; p=<0.0001; and 16 vs. 32mg: F1,20=18; p=<0.001). On average, amounts consumed per dose period (8, 16, and 32 mg/70kg), were well below those maximally allowed (i.e., 96, 192, and 384 mg per 70 kg, respectively), or (71%), (63%), and (53%), respectively. Across all sessions, subjects consumed an average of 393 ± 68 mg of cocaine (208-600mg). In no instance did individuals consume all of cocaine available during a given dose period, but three participants infused 11 (out of the 12 available) doses during five of the dose periods.
Mean plasma concentrations of cocaine were proportional to the amounts consumed (Fig. 1D). Significant main effects of cocaine dose (F2,19=7.86; p=<0.003) and post hoc differences (8 vs. 16mg: F1,19=9; p=<0.007; 8 vs. 32mg: F1,19=15; p=<0.001) or trends (16 vs. 32mg (F1,19=4; p=0.06) were observed.
Subjective effects
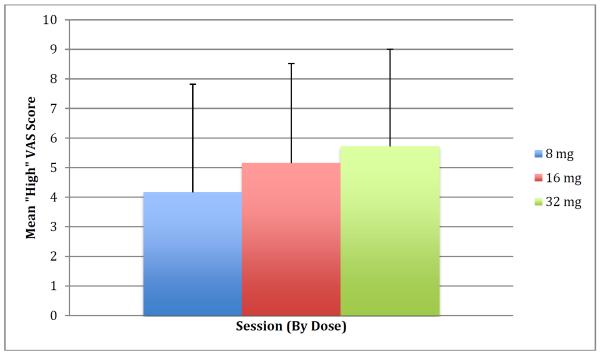
VAS self-reported ratings of cocaine-induced subjective effects showed significant main effects for euphoria (i.e., “I feel high”; dose: F2,373=3.7, p=0.03; time: F12,373=2.0, p=0.02) and for nicotine craving (“I want nicotine”; time: F12,373=2.19, p=0.01), but not for other subjective effects measures. The VAS self-rating changes above baseline were significant for “I need cocaine” (F3,10=13.6, p=<0.001), “I feel high” (ATS=20.1, num df=2.33, p=<0.0001), “I feel hungry” (ATS=4.21, num df=.167, p=0.02), “I need nicotine (ATS=4.31, num df=2.15, p=0.01), “I feel talkative” (ATS=6, num df=1.81, p=<0.01, and trends for “I feel stimulated” (ATS=2.77, num df=1.48; p=0.08) and “I feel tongue-tied” (ATS=2.55, df=2.01, p=0.08). Averaged data for “high” are depicted graphically in Fig. 3.
Fig. 3.
Mean ±SD visual analog scale (VAS; 0=not at all, 10=most ever) self-reported ratings (every 5 min) of “ I feel high” during the 1 hour cocaine self-administration. A significant main effect of cocaine dose session (p=0.03) and time (p=0.02) with “high” was observed.
Discussion
The current study builds upon our group’s prior work by consolidating previously validated, multi-day methods of self-regulated cocaine administration into a single-day design. Procedural innovations were well tolerated, no serious adverse events were observed (Sughondhabirom et al 2005; Lynch et al 2006; Morgan et al 2006; Kalayasiri et al 2007b), and valid dose-response relationships for cardiovascular function (HR), self-administration (responses, infusions, interinfusion intervals, cocaine consumption, and plasma cocaine), and subjective effects (“high”) were retained. As such, these procedures show promise and may lend themselves more practically to medication development efforts involving multi-dose drug (including same-day placebo-controlled) designs.
In comparison to our prior protocols, a principal advantage of our revised methods is the potential to obtain comparable research outcomes at considerably lower research burden to human subjects. Beyond cumulative days of study participation (e.g., four vs. one), such benefits also extend to lesser cumulative cocaine exposures (down from 1112 to 728 mg/70 kg) and total session durations (down from 15 to 6 hours). Importantly, the corresponding increases in the daily quantities of cocaine consumed (up from 384 to 728 mg/70kg) and individual session times (up from 3 to 6 hours) were well tolerated by subjects (i.e., no subjects discontinued participation as a result of the prolonged session duration/larger cumulative cocaine doses). The latter finding is not surprising, however, in light of the doses and durations of cocaine use typically reported by experienced users (including our own) outside of the laboratory.
Consistent with prior human and animal work (e.g., Lynch and Carroll 2001; Pickens and Thompson 1968; Tsibulsky and Norman 1999), including our own (Sughondhabirom et al. 2005; Lynch et al 2006), the current one-day paradigm replicated findings that experienced cocaine users self-regulate their cocaine administration behavior in a highly dose-dependent fashion. Specifically, robust effects of cocaine dose were found for all measures of self-administration behavior, including responses, infusions, and interinfusion intervals. Moreover, total cocaine intake and plasma cocaine concentrations also varied significantly as a function of dose. Taken together, these findings verify that the pharmacological validity of our methods is retained in the context of the shortened, fully randomized, one-day design.
In contrast to self-administration measures, dose-related changes in cocaine-induced cardiovascular responses and subjective effects were less robustly detected by our revised procedures. For example, among cardiovascular outcomes, only HR showed significant effects of cocaine (dose-by-time interaction). This stands in contrast to results obtained using our original multi-day design (Sughondhabirom et al. 2005; Lynch et al 2006). Similarly, among cocaine-induced subjective effects, only euphoria (“high”) was unequivocally shown to vary in a dose-dependent fashion. Thus, for example, our current procedures failed to detect dose-dependent changes in “paranoia,” a measure consistently shown to be dose-responsive in our prior work. Importantly, clear and unequivocal differences in vital signs (HR, SBP, DBP) and subjective effects (in five VAS ratings) were found in comparison to baseline conditions.
Several design features may have lead to the lesser sensitivity of our revised procedures for the detection of dose-related cardiovascular and subjective effects. To keep overall session duration at a minimum, we allowed only 20 minutes between cocaine safety/eligibility and each cocaine self-administration period. Longer cocaine-free periods may be required to allow elevations in vital signs and/or subjective effects to return (more closely) to baseline levels. Similarly, mechanisms such as acute tolerance may also have contributed to attenuated dose-response relationships. Prior work has clearly shown that even a single cocaine dose can influence responses to a second, within-session administration (Fischman et al., 1985), including a relative plateauing of such responses compared to escalating plasma cocaine levels (a finding present in our sample, as well; analyses not shown) (Foltin and Haney, 2000). We specifically considered the possibility of acute tolerance, in addition to other potentially confounding factors (e.g., carryover effects), in our multi-dose paradigm, employing a fully-randomized design as a result (i.e., so that potentially attenuating effects of acute tolerance on ascending dose schedules in some individuals might be offset, for example, by potentially exaggerating effects of acute tolerance on responses to descending dose schedules in others). Despite the strengths of such a design, such effects may have nonetheless contributed to an increased variance in cardiovascular and/or subjective effects data across doses/subjects and, in turn, diminished statistical power for detecting these.
Nonetheless, it is notable that measures of self-administration, HR, and euphoria (“high”) were robust to such features. Outcomes such as “paranoia”, however, appear to be less temporally responsive to cocaine, taking longer to build and longer to wear-off. Certainly, one-hour periods of self-administration (a reduction from 2 hours per dose) may have resulted in lesser sensitivity (although session duration was determined based on analyses of extensive prior self-administration data, suggesting that this is less likely). Finally, we cannot exclude the possibility that differences exist between the current cohort and those of our prior studies (e.g., this may be a factor in our negative “paranoia” findings, where only 2 of our 11 subjects or 18% endorsed VAS ratings ≥5, in contrast to 43% of those in a prior study)(Kalayasiri et al 2007b).
In conclusion, the current study demonstrates the feasibility of a single-day design allowing for the self-regulated administration of cocaine that is safe, well tolerated, and pharmacologically valid. As piloted, these methods reproduced established dose response relationships for drug self-administration and euphoria. Future procedural innovations may ultimately improve the method’s sensitivity for other dose-dependent measures. Taken together, the current one-day design is promising and may have considerable advantages over our prior methods when considerations of research burden to human subjects and/or medication development (including dose-findings) are involved.
Highlights maximum 85 characters, including spaces, per bullet point.
We examine whether a full cocaine dosing range can be studied in humans in one day
Procedures were well tolerated by participants with no significant adverse events noted
Significant dose-dependent changes in measures of cocaine self-administration were found
Changes in cardiovascular responses and subjective effects were less robustly detected
The data support the safety, tolerability and validity of our single-day design
Acknowlegements
We would like to thank the staff of the Clinical Neuroscience Research Unit (CNRU) at CMHC and the Hospital Research Unit (HRU) in YNHH. This work was supported by the National Institute on Drug Abuse (NIDA; K24 DA017899; R03 DA025176; RTM; P20 DA027844; MNP), the National Institute of Mental Health (NIMH; T32 MH019961; DM/RTM), Yale Center for Clinical Investigation (YCCI) Pilot Projects Translational and Interdisciplinary Research and the Department of Mental Health and Addiction Services of the State of Connecticut.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angarita G, Pittman B, Gueorguieva R, Kalayasiri R, Lynch WL, Sughondhabirom A, Morgan PT, Malison RT. Regulation of cocaine self-administration in humans: Lack of evidence for loading and maintenance phases. Pharmacology, Biochemistry and Behavior. 2010;95:51–55. doi: 10.1016/j.pbb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Brunner E, Domhof S, Langer F. Nonparametric analysis of longitudinal data in factorial experiments. Wiley; New York, NY: 2002. [Google Scholar]
- Collins ED, Vosburg SK, Ward AS, Haney M, Foltin RW. Memantine increases cardiovascular but not behavioral effects of cocaine in methadone-maintained humans. Pharmacol. Biochem. Behav. 2006;83(1):47–55. doi: 10.1016/j.pbb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Evans SM, Walsh SL, Levin FR, Foltin RW, Fischman MW, Bigelow GE. The effects of flupenthixol on the subjective and cardiovascular effects of intravenous cocaine in humans. Drug Alcohol Depend. 2001;64:271–283. doi: 10.1016/s0376-8716(01)00129-6. [DOI] [PubMed] [Google Scholar]
- Fischman M, Schuster C, Resnekov L, Schick J, Krasnegor N, Fennell W, Freedman D. Cardiovascular and subjective effects of intravenous cocaine administration humans. Arch Gen Psychiatry. 1976;33:983–989. doi: 10.1001/archpsyc.1976.01770080101010. [DOI] [PubMed] [Google Scholar]
- Fischman M, Schuster C, Rajfer S. A comparison of the subjective and cardiovascular effects of cocaine and procaine in humans. Pharmacol Biochem Behav. 1983a;18:711–716. doi: 10.1016/0091-3057(83)90011-4. [DOI] [PubMed] [Google Scholar]
- Fischman M, Schuster D, Hatano Y. A comparison of the subjective and cardiovascular effects of cocaine and lidocaine in humans. Pharmacol Biochem Behav. 1983b;18:123–127. doi: 10.1016/0091-3057(83)90261-7. [DOI] [PubMed] [Google Scholar]
- Fischman MW. Relationship between self-reported drug effects and their reinforcing effects: studies with stimulate drugs. Testing for abuse liability of drugs in humans. 1989. NIDA Research Monograph, Number 92. [PubMed]
- Fischman MW, Foltin RW, Nestadt G, Pearlson GD. Effects of desipramine maintenance on cocaine self-administration by humans. J Pharmacol Exp Ther. 1990;253:760–770. [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Self-administration of cocaine by humans: a laboratory perspective. Ciba Found Symp. 1992;166:165–180. doi: 10.1002/9780470514245.ch10. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Smoked and intravenous cocaine in humans: acute tolerance, cardiovascular and subjective effects. J Pharmacol Exp Ther. 1991a;257(1):247–261. [PubMed] [Google Scholar]
- Foltin R, Fischman M. Assessment of abuse liability of stimulant drugs in humans: a methodological survey. Drug Alcohol Depend. 1991b;28:3–48. doi: 10.1016/0376-8716(91)90052-z. [DOI] [PubMed] [Google Scholar]
- Foltin R, Fischman M. Methods for the assessment of abuse liability of psychomotor stimulants and anorectic agents in humans. Br J Addict. 1991c;86:1633–1640. doi: 10.1111/j.1360-0443.1991.tb01758.x. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Levin FR. Cardiovascular effects of cocaine in humans: laboratory studies. Drug Alcohol Depend. 1995;37(3):193–210. doi: 10.1016/0376-8716(94)01085-y. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Ward AS, Haney M, Hart CL, Collins ED. The effects of escalating doses of smoked cocaine in humans. Drug Alcohol Depend. 2003;70(2):149–157. doi: 10.1016/s0376-8716(02)00343-5. [DOI] [PubMed] [Google Scholar]
- Grasing K, Mathur D, Newton TF, DeSouza C. Donepezil treatment and the subjective effects of intravenous cocaine in dependent individuals. Drug Alcohol Depend. 2010;107:69–75. doi: 10.1016/j.drugalcdep.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Haberny K, Walsh S, Ginn D, Wilkins J, Garner J, Setoda D, Bigelow G. Absence of acute cocaine interactions with the MAO-B inhibitor selegiline. Drug Alcohol Depend. 1995;39:55–62. doi: 10.1016/0376-8716(95)01137-n. [DOI] [PubMed] [Google Scholar]
- Haney M, Foltin RW, Fischman MW. Effects of pergolide on intravenous cocaine self-administration in men and women. Psychopharmacology. 1998;137:15–24. doi: 10.1007/s002130050588. [DOI] [PubMed] [Google Scholar]
- Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW. Effect of a selective dopamine D1 agonist (ABT-431) on smoked cocaine self-administration in humans. Psychopharmacology. 1999;143:102–110. doi: 10.1007/s002130050925. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology. 2001;155:330–337. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- Hart C, Haney M, Vosburg S, Comer S, Gunderson E, Foltin RW. Modafinil attenuates disruptions in cognitive performance during simulated night-shift work. Neuropsychopharmacology. 2006;31:1526–1536. doi: 10.1038/sj.npp.1300991. [DOI] [PubMed] [Google Scholar]
- Hart C, Haney M, Vosburg S, Rubin E, Foltin R. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33:761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Kalayasiri R, Sughondhabirom A, Gueorguieva R, Coric V, Lynch WJ, Morgan PT, Cubells JF, Malison RT. Self-reported paranoia during laboratory ‘binge’ cocaine self-administration in humans. Pharmacol Biochem Behav. 2006;83:249–256. doi: 10.1016/j.pbb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Kalayasiri R, Morgan P, Pittman B, Gueorguieva R, Coric V, Bhagwagar Z, Cubells J, Malison RT. 69th College on Problems of Drug Dependence. Quebec, Canada: 2007a. Disulfiram enhances paranoia during “binge” cocaine self-administration. [Google Scholar]
- Kalayasiri R, Sughondhabirom A, Gueorguieva R, Coric V, Lynch WJ, Lappalainen J, Gelernter J, Cubells JF, Malison RT. Dopamine beta-hydroxylase gene (DbetaH) −1021C→T influences self-reported paranoia during cocaine self-administration. Biol Psychiatry. 2007b;61:1310–3. doi: 10.1016/j.biopsych.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clin Psychopharmacol. 2001;9:131–43. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Sughondhabirom A, Pittman B, Gueorguieva R, Kalayasiri R, Joshua D, Coric V, Morgan PT, Malison RT. A paradigm to investigate the regulation of cocaine self-administration in human cocaine users: A randomized trial. Psychopharmacology (Berl) 2006;185:306–314. doi: 10.1007/s00213-006-0323-5. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Kalayasiri R, Sughondhabirom A, Pittman B, Coric V, Morgan PT, Malison RT. Subjective responses and cardiovascular effects of self-administered cocaine in cocaine-abusing men and women. Addict Biol. 2008;13:403–10. doi: 10.1111/j.1369-1600.2008.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang D-R, Huang Y, Perez A, Frankel WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Cocaine dependence and D2 receptor availability in the functional subdivisions of the striatum: Relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29(6):1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper T, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: Markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Matuskey D, Pittman B, Malison R, Morgan PT. A Multistudy Analysis of the Effects of Cocaine Abstinence on Sleep. Drug and Alcohol Dependence. 2011;115(1-2):62–6. doi: 10.1016/j.drugalcdep.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Malison RT. Pilot study of lorazepam and tiagabine effects on sleep, motor learning, and impulsivity in cocaine abstinence. Am J Drug Alcohol Abuse. 2008;34:692–702. doi: 10.1080/00952990802308221. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep dependent procedural learning and vigilance in chronic cocaine users: evidence for occult insomnia. Drug Alcohol Dependence. 2006;82:238–49. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Newton TF, Kalechstein A, Beckson M, Bartzokis G, Bridge TP, Ling W. Effects of selegiline pretreatment on response to experimental cocaine administration. Psychiatry Res. 1999;87:101–106. doi: 10.1016/s0165-1781(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Oliveto A, McCance-Katz FE, Singha A, Petrakis I, Hameedi F, Kosten TR. Effects of cocaine prior to and during Bupropion maintenance in cocaine-abusing volunteers. Drug and Alcohol Dependence. 2001;63(2):155–167. doi: 10.1016/s0376-8716(00)00198-8. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Morgan PT, Malison RT, Hart CL, Edgar C, Walker M, Stickgold R. Cocaine users differ from normals on cognitive tasks which show poorer performance during drug abstinence. Am J Drug Alcohol Abuse. 2008;34:109–21. doi: 10.1080/00952990701764821. [DOI] [PubMed] [Google Scholar]
- Penetar DM, Looby AR, Su Z, Lundahl LH, Eros-Sarnyai M, McNeil JF, Lukas SE. Benztropine pretreatment does not affect responses to acute cocaine administration in human volunteers. Hum Psychopharmacol. 2006;21(8):549–59. doi: 10.1002/hup.810. [DOI] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther. 1968;161:122–9. [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Rotheram-Fuller E, De La Garza R, Mahoney JJ, Shoptaw S, Newton TF. Subjective and cardiovascular effects of cocaine during treatment with amantadine and baclofen in combination. Psychiatry Research. 2007;152:205–10. doi: 10.1016/j.psychres.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Sevak RJ, Hays LR. Cocaine choice in humans during d-amphetamine maintenance. J Clin Psychopharmacol. 2010;30:152–159. doi: 10.1097/JCP.0b013e3181d21967. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Pearlson GD, Wong DF, Marenco S, Dannals RF. PET study of competition between intravenous cocaine and [11C]raclopride at dopamine receptors in human subjects. Am J Psychiatry. 1997;154(9):1209–1213. doi: 10.1176/ajp.154.9.1209. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Poling J, Mooney M, Hatsukami DK. The effect of individual cocaine withdrawal symptoms on outcomes in cocaine users. Addict Behav. 2005;30:1125–1134. doi: 10.1016/j.addbeh.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Sughondhabirom A, Jain D, Gueorguieva R, Coric V, Berman R, Lynch WJ, Self D, Jatlow P, Malison RT. A paradigm to investigate the self-regulation of cocaine administration in humans. Psychopharmacology. 2005;180:436–46. doi: 10.1007/s00213-005-2192-8. [DOI] [PubMed] [Google Scholar]
- Tsibulsky VL, Norman AB. Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res. 1999;839:85–93. doi: 10.1016/s0006-8993(99)01717-5. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Enadoline and butorphanol: evaluation of kappa-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther. 2001;299:147–158. [PubMed] [Google Scholar]