Figure 10.
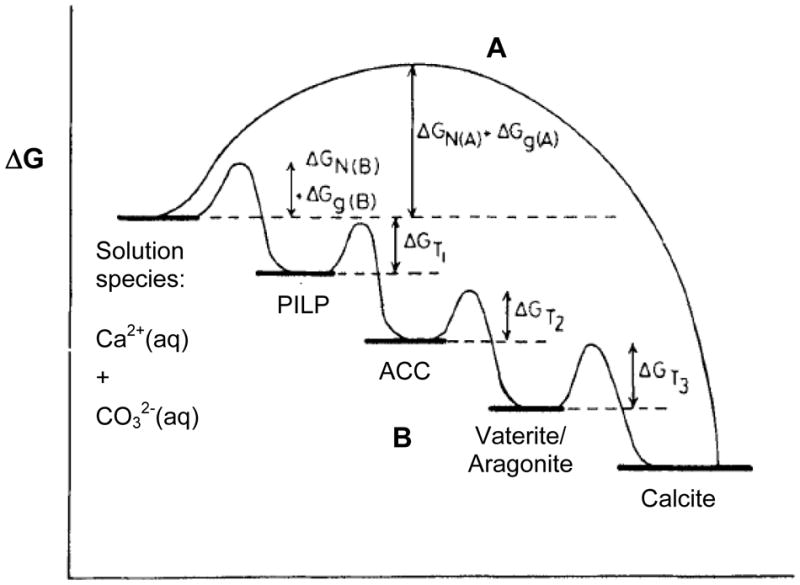
Reaction coordinate diagram representing different pathways to lowering the Gibbs free energy. Pathway A, which I will refer to as the conventional crystallization pathway throughout this manuscript, occurs for mineral precipitation de novo from solution, without passing through any intermediate phase(s) that require subsequent structural modifications. Pathway B demonstrates formation of a crystalline mineral from metastable phase(s) of different crystal structures, or even the amorphous phase. The energy barriers for the pathways differ, where the first step requires a ΔGNucleation + ΔGGrowth, while the subsequent steps only require a ΔGPhase Transformation. Calcium carbonate (CaCO3) is used as an example here, but it should be noted that the energy levels of the different CaCO3 phases and the heights of the energy barriers are not drawn to scale. A variety of pathways are possible, depending on the relative heights of the energy barriers, such as formation of ACC followed by transformation directly to calcite, and so on. Note- I have proposed a new step in the ACC pathway, which consists of a polymer-induced liquid-precursor (PILP) phase being a potential intermediate to the solid ACC phase.
