Figure 48.
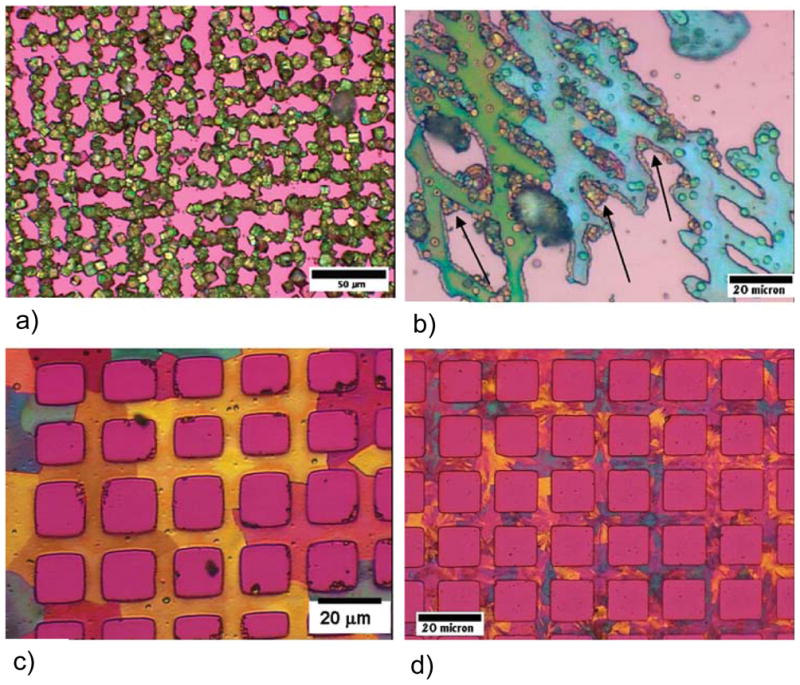
Polarized light micrographs (using first-order red gypsum λ-plate) of calcite templated on patterned SAMs, first printed with COO−-terminated SAMs in a grid pattern, and then CH3-terminated SAMs were coated on the remaining square areas. (a) Patterned rhombohedral polycrystals of calcite formed by the conventional solution crystallization. (b) Patterned films of calcite generated with polymeric process-directing agent. The films are composed of large single-crystalline domains of calcite (e.g., one crystal is aqua blue and the other green), but also contain many late-stage PILP droplets and crystalline aggregates due to the incomplete inhibition of solution crystallization byproducts. Note the preference for adsorption of the late-formed PILP droplets at the edges of the films (arrows). (c) Well-defined patterned film of calcite composed of single-crystalline domains on the order of 10s of microns (regions of uniform retardation color and extinction direction). A lower reaction temperature reduced the number of crystal side-products, but a few small aggregates are still present. (d) Well-defined patterned films of calcite using a combination of polymer and Mg-ion inhibitors. The Mg-ion inhibitor, while eliminating crystal byproducts, also acts as an impurity that causes a more polycrystalline texture in the film. (Reprinted with permission from ref 221. Copyright 2007 American Chemical Society.)
