Abstract
Background
We investigated affect recognition and the impact of emotional valence on working memory (using happy, angry, and neutral faces) in pediatric patients with bipolar disorder (BD) and healthy controls (HC).
Method
Subjects (N=70) consisted of unmedicated patients with BD type I (n=23) and type II (n=16) and matched HC (n=31). All subjects completed tasks of emotion recognition (Chicago Pediatric Emotional Acuity Task; Chicago PEAT) and working memory for happy, angry, and neutral faces (Affective N-Back Memory Task; ANMT).
Results
Compared to HC, BD patients performed significantly more poorly when identifying the intensity of happy and angry expressions on the Chicago PEAT, and demonstrated working memory impairments regardless of the type of facial emotional stimuli. Pediatric BD patients displayed the most impaired accuracy and reaction time performance with negative facial stimuli relative to neutral stimuli, but did not display this pattern with positive stimuli. Only BD type I patients displayed working memory deficits, while both type I and type II patients displayed emotion identification impairments. Results remained significant after controlling for comorbid ADHD and mood state.
Conclusion
Both type I and type II BD youth demonstrate emotion identification deficits. BD youth also demonstrate working memory impairments for facial stimuli irrespective of emotional valence, however, working memory deficits were the most pronounced with negative emotional stimuli. These deficits appear to be specific to BD type I patients, and suggest therefore that a more severe form of illness is characterized by more severe social-cognitive impairment.
Keywords: Pediatric bipolar disorder, ADHD, emotion identification, attention, working memory
Pediatric bipolar disorder (BD) is a debilitating illness characterized by rapid mood swings, significant functional impairment, and chronic morbidity (see Leibenluft & Rich, 2008 and Pavuluri, Birmaher, & Naylor, 2005 for reviews). Deficits in social and interpersonal functioning are core features of the illness, and are thought to reflect disturbances in cognitive and affect processing circuitry (Rich et al., 2008a, 2010; Schenkel et al., 2007, 2008). Significant impairments in attention and working memory (Dickstein et al., 2004; Pavuluri et al., 2006), along with deficits in emotion processing have been documented in BD youth (Rich et al., 2008b; Schenkel et al., 2007), as well as unaffected first-degree relatives (Bora et al., 2009; Brotman et al., 2008a; Doyle et al., 2009). Moreover, disturbances in emotion identification have been found across emotions in pediatric BD patients, and are thought to be an endophenotype for the disorder (Brotman et al., 2008a, 2008b; Rich et al., 2008b).
Recent models of the pathophysiology of pediatric BD suggest abnormalities in frontolimbic circuitry, and point to important functional interactions in emotion processing between higher cortical cognitive areas and regions associated with the evaluation and processing of affective stimuli (Pavuluri et al., 2007). A number of functional magnetic resonance imaging (fMRI) studies of emotion processing in youth with BD have identified abnormal activation in cortical areas such as the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), ventromedial prefrontal cortex (VMPFC) and anterior cingulate cortex (ACC), along with subcortical areas that include the striatum, and most notably the amygdala (Chang et al., 2004; Passarotti, Sweeney, & Pavuluri, 2010, 2011; Pavuluri et al., 2007, 2008, 2010b; Rich et al., 2006). Additionally, studies have documented smaller hippocampal volume among adolescents with BD relative to controls (Bearden et al., 2008). Together, these regions are involved in receiving, attending to, evaluating, and modulating emotional stimuli, including top-down regulation of emotional responses and the appraisal of reward and punishment (Adolphs, 2009; Botvinick et al., 2004; Cools et al., 2002; Packard & Cahill, 2001; Weissman, Perkins, & Woldorff, 2008; Völlm et al.). Additionally, Rich et al., (2008a) reported reduced connectivity between the left amygdala and right posterior cingulate/precuneus and right parahippocampal gyrus, indicating that working memory may be an important factor associated with emotion identification deficits in BD youth.
Given the complexity and interconnectivity of emotion centers and higher cortical regions, it is likely that emotion processing impairments and cognitive dysfunction among BD youth are interrelated and bidirectional in nature. For example, the excessive emotional reactivity characteristic of a manic/acute state may have a negative effect on cognitive functioning, and deficient executive control could reduce top-down modulation of the intensity and duration of emotional experiences (Pavuluri et al., 2008). Past behavioral and imaging studies are consistent with this model. For example, Rich et al., (2005) reported more impaired attentional performance among BD youth when placed in emotionally challenging and frustrating contexts. In addition, our group recently found that BD adolescents show reduced activation in higher cortical centers (e.g., DLPFC, VLPFC) when exposed to emotional stimuli, such as emotional faces or words, and increased activation in the amygdala, suggesting that cortical centers become less active and fail to provide adequate top-down regulation over subcortical emotion areas (Passarotti et al., 2010, 2011; Pavuluri et al., 2007, 2008). Deficient top-down modulation has been observed for positive and negative emotional stimuli among pediatric BD patients, however, findings appear to be more robust for negative ones, suggesting a heightened sensitivity for negative contexts (Passarotti et al., 2011; Pavuluri et al., 2008; Pavuluri, Passarotti, Fitzgerald, Weqbreit, & Sweeney, 2012).
Understanding the interface between emotional and cognitive functioning in youth with BD can help to elucidate the underlying mechanisms associated with the disorder, and may also help to distinguish between clinical phenotypes in pediatric BD. To date, there has been limited research on the relationships between attention and memory, and emotion processing in pediatric BD. This is surprising as a number of independent investigations have consistently documented working memory and emotion processing deficits in acute and euthymic pediatric BD patients (Pavuluri et al., 2006; Rich et al., 2008b; Schenkel et al., 2007, 2012). Cognitive and affective systems work in tandem, and the successful interaction between these systems is paramount for adaptive behavior (Passarotti et al., 2011; Pavuluri et al., 2010b). The ability to attend to, store, and process information in short-term memory is essential for successful day-to-day activities. Adaptive behavior also requires the ability to accurately process and respond to emotional cues. Given the rich interaction of these systems, we sought to investigate affect identification and working memory for emotional faces in youth with BD type I (BD I) and type II (BD II) and matched healthy control (HC) subjects. Consistent with past work, we hypothesized that BD youth would perform significantly more poorly compared to healthy subjects when identifying both happy and angry emotional expressions. In addition, we hypothesized that compared to healthy subjects, BD youth would show difficulty attending to and recalling positive and negative emotional facial stimuli, and that these deficits would be most pronounced with negative stimuli. Given the severity of manic symptoms in BD I versus BD II patients, we also hypothesized that youth with BD I would show the most impaired performance on the emotion processing and working memory tasks.
Method
Subjects
BD subjects were recruited from the Pediatric Mood Disorders Clinic at the University of Illinois at Chicago (UIC). The Institutional Review Board approved the study. Verbal or written assent was provided by all children in addition to the written informed consent by parents. The BD I (n=23) and BD II (n=16) unmedicated patients and HC (n=31) were between the ages of 8 and 18 years (Mean age=13.39, SD=2.98), and were recruited to be similar on age, sex (36 males), parental socio-economic status, and intelligence as assessed by the 2-subtest version of the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler et al., 1998). Inclusion criteria for the BD group were a current Diagnostic and Statistical Manual of Mental Disorders, Text Revision (DSM-IV-TR; American Psychiatric Association, 2000) diagnosis of BD I, mixed (n=8) or manic (n=15) state, or BD II, hypomanic (n=8) or depressed (n=8) state, and medication free for at least one week prior to testing. In the BD group, 14 subjects (41%) had a comorbid diagnosis of attention deficit hyperactivity disorder (ADHD). Healthy comparison subjects were euthymic with Young Mania Rating Scale (YMRS; Young et al., 1978) scores of ≤ 8 and Child Depression Rating Scale (CDRS-R; Poznanski et al., 1985) scores of ≤ 40. None of the subjects in the healthy comparison group met DSM IV-TR (American Psychiatric Association, 2000) criteria for any major psychiatric disorder (see Table 1 for demographic and clinical data).
Table 1.
Demographic and clinical characteristics of the pediatric bipolar disorder I (BD I) and II (BD II) and healthy comparison (HC) subjects. Means, standard deviations (SD), percentages, and significance values are presented below.
| HC | BD I | BD II | Analysis | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | F(p) | |
| Variables | ||||
| Age (years) | 13.00 (3.40) | 12.61 (3.50) | 14.56 (2.03) | 1.93 (.15) |
| Socioeconomic statusa | 1.90 (0.91) | 1.78 (0.74) | 2.06 (0.93) | 0.50 (.61) |
| YMRS | 1.68 (2.68) | 24.17 (8.02) | 14.00 (6.12) | 84.78 (<.0001) |
| CDRS-R | 19.48 (2.41) | 51.52 (15.55) | 54.44 (16.73) | 56.17 (<.0001) |
| WASI IQ | 109.52 (13.91) | 105.43 (15.69) | 100.25 (13.16) | 2.29 (.11) |
| N (%) | N (%) | N (%) | χ2 (p) | |
|---|---|---|---|---|
| Sex | 0.53 (.77) | |||
| Male | 17 (55%) | 12 (52%) | 7 (44%) | |
| Female | 14 (45%) | 11 (48%) | 9 (56%) | |
| Race | 2.43 (.28) | |||
| Caucasian | 15 (48%) | 16 (70%) | 9 (56%) | |
| Other | 16 (52%) | 7 (30%) | 7 (44%) |
Rated with Hollingshead Index of Social Position
Each child and at least one of their parents were interviewed using the Washington University St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS; Geller et al., 1998) supplemented by the episode characterization of bipolar disorder from the KSADS- Present and Lifetime version (KSADS-PL; Kaufman et al., 1997), along with a comprehensive clinical interview. Clinical information from all available sources was combined to provide a consensus clinical diagnosis. The WASH-U-KSADS interviews as well as the YMRS and CDRS-R were completed by trained doctoral level raters who had no knowledge of social cognition test performance. Live diagnostic interviews of ten cases were coded by three research assistants and MNP to establish inter-rater reliability. By Cohen's Kappa, reliability of diagnoses was 0.96 between the raters. Exclusion criteria for the entire sample were active substance abuse, serious medical problems, IQ <70, or the presence of another current DSM-IV-TR axis I diagnosis that required psychiatric intervention (pharmacotherapy or psychotherapy) with the exception of ADHD.
Procedure
Measures of Emotion Processing and Working Memory
Chicago Pediatric Emotional Acuity Task (Chicago PEAT)
The Chicago PEAT is a modified version of the PEAT (Erwin et al., 1992), and measures the ability to identify happy and angry facial expressions that range from neutral to extreme in emotional intensity. It requires individuals to rate 40 color pictures of child and adult faces along a 7-point continuum of emotional intensity (very happy, moderately happy, slightly happy, neutral, slightly angry, moderately angry, and very angry). Facial stimuli were standardized color photographs of varying emotional intensity from the Diagnostic Analysis of Nonverbal Accuracy Scale (DANVA; Nowicki & Duke, 1994). Past studies using this measure have demonstrated facial emotion processing abnormalities among pediatric BD patients (McClure et al., 2005). The task is self-paced, response driven, and takes approximately 4 to 5 minutes to complete Outcome variables are total correct, total incorrect, and mean reaction time for happy and angry stimuli.
Affective N-Back Memory Task (ANMT; Passarotti et al., 2010, 2011; Pavuluri et al., 2012)
The ANMT measures working memory for happy, angry, and neutral facial stimuli, and has been shown to effectively differentiate the neuropathophysiology of pediatric BD and ADHD patients (Passarotti et al. 2010). It is comprised of two separate working memory tasks – the ANMT-Positive and the ANMT-Negative. Each task is a two-back memory task, and uses a block design with faces as the targets. There are a total of 80 trials each for the ANMT-Positive (40 happy, 40 neutral) and ANMT-Negative (40 angry; 40 neutral) for a total of 160 trials. Within each task, facial stimuli are presented in a blocked format, and blocks are presented in a counterbalanced pseudorandom sequence. In each block there are 8 target faces. Each face is presented for three seconds, and participants are asked to respond if the target face appeared two faces back in the series. Distracter faces were not used more than once. The face stimuli were taken from the Gur et al., (2002) database, and were balanced by age, gender, and race. True-positives, false-positives, and mean reaction times were scored for each task.
Statistical Analysis
To provide a standard metric for comparison of deficits across the emotion processing tasks, scores were standardized to z scores based on the means and standard deviations from the healthy comparison group (Herbener et al., 2005; Pavuluri et al., 2006; Schenkel et al., 2007). On the Chicago PEAT, we subtracted total incorrect response scores from total correct response scores in order to form ‘emotion identification composites’ for both the happy and angry conditions. Total scores for each condition were calculated for the ANMT-Positive and ANMT-Negative by subtracting true positive from false positive scores (i.e., z scores for the happy and neutral conditions of the ANMT-Positive, and z scores for the angry and neutral conditions of the ANMT-Negative).
Mixed model multivariate analyses of variance (MANOVA) using Wilks’ lambda’ were used to assess for group differences between the BD and HC groups on the Chicago PEAT (3 × 2) and the ANMT-Positive (3 × 2) and ANMT-Negative (3 × 2), with diagnostic group as the between-subjects variable and emotional condition as the within-subjects variable, with follow-up post-hoc pairwise comparisons to assess for group differences between the BD I and II groups and the HC group. Where there were significant interaction effects, paired sample t-tests were used to examine mean differences between affective conditions for the BD I and II groups separately. To control for comorbid ADHD and mood state in the BD groups, separate 3 × 2 MANCOVAs were done with ADHD and mood state as covariates. Finally, associations between performance on the Chicago PEAT and ANMT, and level of symptomatology (CDRS-R and YMRS scores) and age were analyzed separately for the BD and HC groups using Pearson Correlation Coefficients.
Results
As expected, there were significant differences between the three groups on the YMRS (F(2,67)=103.54, p<.0001) and CDRS-R (F(2,67)=66.25, p<.0001) (see Table 1 for means and standard deviations). Both BD groups had higher YMRS scores compared to controls (ps<.0001), and BD I patients had significantly higher YMRS scores compared to BD II patients (p<.0001). Similarly, both BD groups had significantly higher CDRS-R scores compared to controls (ps<.0001), however, the BD I and II groups did not differ from each other on CDRS-R severity (p=.74).
Group Comparisons on the Chicago PEAT
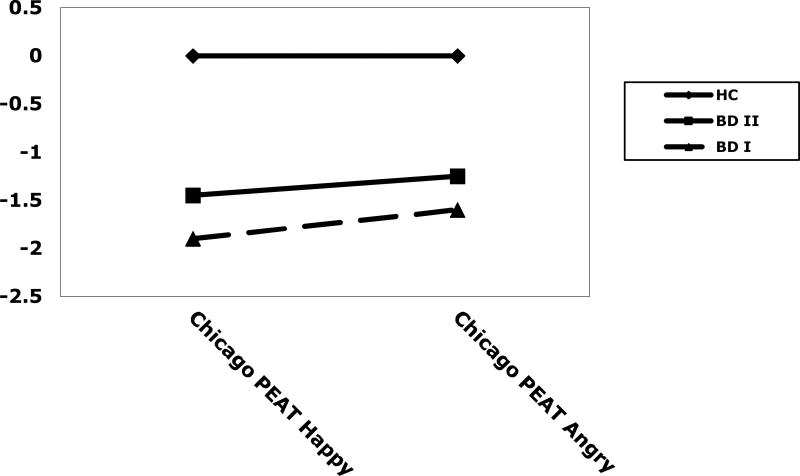
For accuracy scores (correct responses) a 3 (group) × 2 (condition) mixed model MANOVA indicated a significant main effect of group (F(2, 67)=8.43, p<001), but no significant effect of condition (F(1, 67)=0.27, p=.61), or group × condition interaction (F(2,67)=0.10, p=.90). Post-hoc Tukey Honestly Significant Different (HSD) tests indicated that the control group had more correct responses than the BD I group (p=.001), and the BD II group (p<.05). The BD I and BD II groups did not differ from each other (p=.75). However, group differences were not significantly affected by emotion, in that the magnitude of the group difference was not significantly different across the two emotion conditions. See Table 2 and Figure 1.
Table 2.
Means, standard deviations (SD), and group comparisons on the Chicago Pediatric Emotional Acuity Task (Chicago PEAT) and the Positive and Negative Affective N-Back Memory Task (ANMT) for healthy comparison (HC) and pediatric bipolar disorder I (B DI) and II (B DII) subjects. Average z scores for correct responses are presented.
| HC | BD I | BD II | Group Comparisons | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Chicago PEAT | ||||
| Happy | 0.00 (1.00) | -1.90 (1.79) | -1.45 (2.08) | HC>BD I*** |
| HC>BD II* | ||||
| BD I=BD II | ||||
| Angry | 0.00 (1.00) | -1.57 (2.51) | -1.25 (2.49) | HC>BD I*** |
| HC>BD II* | ||||
| BD I=BD II | ||||
| ANMT-Positive | ||||
| Happy | 0.00 (1.00) | -2.10 (3.41) | -0.68 (2.46) | HC>BD I** |
| HC=BD II | ||||
| BD I=BD II | ||||
| Neutral | 0.00 (1.00) | -1.80 (2.62) | -1.13 (2.71) | HC>BD I** |
| HC=BD II | ||||
| BD I=BD II | ||||
| ANMT-Negative | ||||
| Angry | 0.00 (1.00) | -2.48 (3.78) | -0.86 (1.28) | HC>BD I*** |
| HC=BD II | ||||
| BD I< BD IIt | ||||
| Neutral | 0.00 (1.00) | -1.32 (2.37) | -0.44 (1.31) | HC>BD I** |
| HC=BD II | ||||
| BD I=BD II | ||||
<.1
p<.05
p<.01
p≤.001
Figure 1.
z scores on the Chicago Pediatric Emotional Acuity Task (Chicago PEAT) of healthy comparison subjects (HC) and bipolar disorder type I (BD I) and type II (BD II) subjects.
For reaction time, there were no significant main effects of group (F(2,67)=0.13, p=.88) or condition (F(1,67)=2.45, p=.12), and there was a trend toward a non-significant group × condition interaction (F(2,67)=2.68, p=.08).
Secondary Analyses: Chicago PEAT
For accuracy, findings did not change using a 3 × 2 MANCOVA with ADHD comorbidity as a covariate. Specifically, there was a main effect of group (F(2,66)=5.51, p<.005), and no main effect of condition (F(1,66)=0.22, p=.64), or group × condition interaction (F(2,66)=0.09, p=.92).
Similarly, accuracy findings remained the same with mood state as a covariate, with a significant main effect of group (F(2,66)=3.61, p<.05), and no significant main effect of condition (F(1,66)=0.74, p=.39) or group × condition interaction (F(2,66)=0.42, p=.66).
Group Comparisons on the ANMT
ANMT-Positive
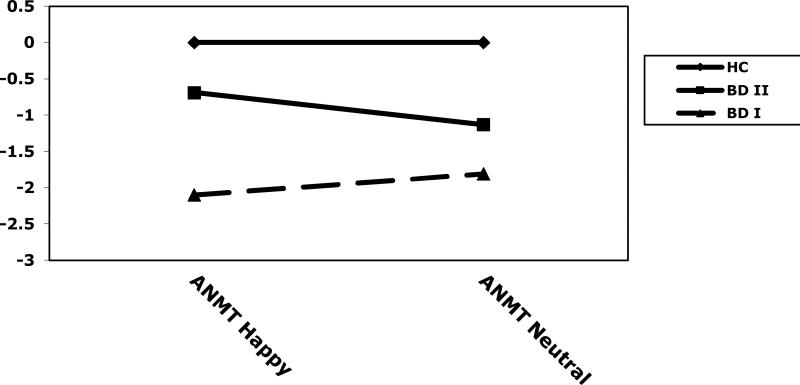
For accuracy on the ANMT-Positive, a 3 × 2 MANOVA indicated a significant main effect of group (F(2, 67)=5.32, p<.01), with the BD group displaying fewer correct responses than the HC group. However, the main effect of condition (F(1, 67)=0.12, p=.73) and group × condition interaction were not significant (F(2, 67)=2.05, p=.14).
For reaction time (3 × 2 MANOVA), there was a non-significant trend toward a main effect of group (F(2,67)=2.50, p=.09). The main effect of condition (F(1,67)=0.32, p=.58) and group × condition interaction (F(2,67)=0.56, p=.57) were not significant.
Secondary Analyses: ANMT-Positive
For accuracy, with ADHD comorbidity included as a covariate (3 × 2 MANCOVA), there was a trend toward a significant main effects of group (F(2,66)=2.54, p=.08), and no main effect of condition (F(1,66)=0.04, p=.84) or group × condition interaction (F(2,66)=1.98, p=.15). Similar results were found for reaction time while controlling for ADHD comorbidity, with no significant main effects of group (F(2,66)=1.28, p=.29) or condition (F(1,68)=0.00, p=.97), and no group × condition interaction (F(2,66)=0.24, p=.79).
For accuracy with mood state as a covariate, there was a main effect of group (F(2,66)=4.62, p<.05) and condition (F(1,66)=4.07, p<.05), and a significant interaction (F(2,66)=3.73, p<.05) indicating that the magnitude of the group difference was significantly different across the two conditions. Post-hoc HSD tests indicated that, on the happy condition of the ANMT-Positive, the HC group had more correct responses than the BD I group (p<.01). There were no significant differences between the BD II group and the HC group, or the BD I and II groups (ps>.05). On the neutral condition of the ANMT-Positive, the HC group had more correct responses than the BD I group (p<.01). There were no significant differences between the BD II group and the HC group, or the BD I and II groups (ps>.05). When examining the BD I and II groups independently, there were no significant differences between mean scores on the neutral versus the happy conditions for BD I (t(22)=1.14, p=.27) or BD II patients (t(15)=1.57, p=.14). See Table 2 and Figure 2.
Figure 2.
z composite scores on the Affective N-Back Memory Task - Positive (happy and neutral conditions) of healthy comparison subjects (HC) and bipolar disorder type I (BD I) and type II (BD II) subjects.
For reaction time with mood state as a covariate, there was a trend toward a significant main effect of group (F(2,66)=2.46, p=.09), and no significant main effect of condition (F(1,66)=0.07, p=.79), or group × condition interaction (F(2,66)=0.06, p=.81).
ANMT-Negative
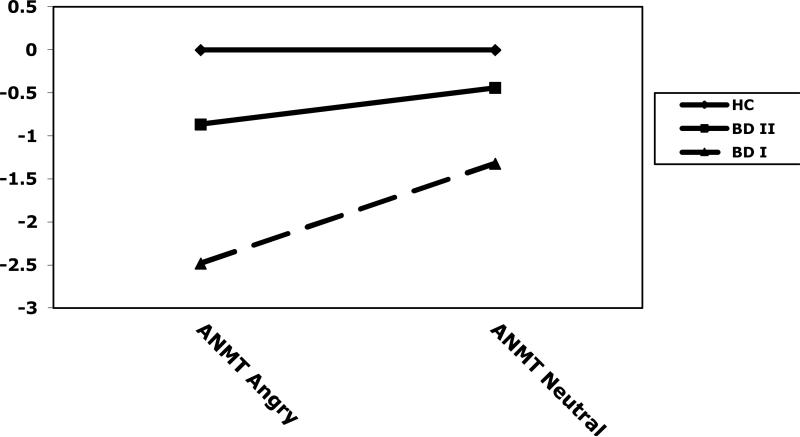
For accuracy on the ANMT-Negative, there was a significant main effect of group (F(2,67)=6.48, p<.01), and condition (F(1,67)=11.53, p<.001), and a significant group × condition interaction (F(2,67)=5.66), p<.01). Post-hoc Tukey HSD tests indicated that, on the angry condition of the ANMT-Negative, the HC group had more correct responses than the BD I group (p<.001), and there was a non-significant trend for the BD II group to perform better than the BD I group (p<.10). The BD II and HC groups did not differ from each other (p=.46). On the neutral condition of the ANMT-Negative, the control group exhibited more correct responses than the BD I group (p<.01), and there were no significant differences between the BD I group and the BD II group (p=.23) or between the BD II and HC groups (p=.66). When examining BD subtype groups independently, both BD I (t(22)=2.69, p<.05) and BD II (t(15)=3.41, <.01) patients had higher accuracy scores on the neutral compared to the negative condition. See Table 2 and Figure 3.
Figure 3.
z composite scores on the Affective N-Back Memory Task - Negative (angry and neutral conditions) of healthy comparison subjects (HC) and bipolar disorder type I (BD I) and type II (BD II) subjects.
On reaction time, there was a significant main effects of group (F(2,67)=4.64, p<.05), and condition (F(1,67)=11.63, p<.001), and a significant interaction (F(2,67)=3.91), p<.05). Post-hoc Tukey HSD tests indicated that, on the angry condition of the ANMT-Negative, the BD I group had significantly slower reaction times than the HC group (p<.001), and there was a non-significant trend for the BD I group to perform slower than the BD II group (p<.10). The BD II and HC groups did not differ from each other (p=.50). There were no significant group differences on the neutral condition (ps>.05). BD I patients had significantly slower reaction times on the neutral compared to the negative condition (t(22)=3.30, p<.005). There was no significant difference between the two conditions for BD II patients (t(15)=1.57, p=.14).
Secondary Analyses: ANMT-Negative
With ADHD included as a covariate (3 × 2 MANCOVA), the accuracy findings did not change. The main effects of group (F(2,66)=3.62, p<.05) and condition (F(1,66)=14.41, p<.0001), and the group × condition interaction (F(2,66)=7.12, p<.005) remained significant. A MANCOVA examining reaction time with ADHD as a covariate was also consistent, with significant main effects of group (F(2,66)=3.08, p<.05) and condition (F(1,66)=10.75, p<.01), and a significant group × condition interaction (F(2,66)=3.85, p<.05).
For accuracy with mood state as a covariate, the main effect of group (F(2,66)=6.78, p<.01) and the group × condition interaction (F(2,66)=6.13, p<.005) remained significant, and there was a trend toward a significant main effect of condition (F(2,66)=3.31, p=.07). Similarly, for reaction time, there was a main effect of group (F(2,66)=3.45, p<.05) and a group by condition interaction (F(2,66)=3.26, p<.05), but no main effect of condition (F(2,66)=0.67, p=.80). See Figure 1 for group differences on the Chicago PEAT and ANMT.
Relationships with Manic and Depressive Symptomatology
In an effort to reduce the overall number of analyses, the Happy and Angry subscales of the Chicago PEAT were combined and averaged to form an overall Chicago PEAT score. Similarly, the four subscale scores of the ANMT were combined to form an overall ANMT composite. Correlations between the two composite scores and depressive and manic symptomatology were computed separately for the HC and the BD group. There were no significant associations between YMRS scores or CDRS-R scores and the Chicago PEAT or ANMT scores in the HC group. For BD patients, there were no significant associations between CDRS-R scores and performance on the Chicago PEAT or ANMT. However, higher YMRS scores were associated with fewer correct responses on the ANMT (r=-.37, p<.05). Additionally, higher scores on the Chicago PEAT were associated with higher scores on the ANMT (r=.38, p<.05). In the HC group, older age was significantly correlated with increased accuracy on the ANMT (r=-.43, p<.05), however, there was not a significant association between age and scores on the Chicago PEAT (r=.18, p=.33). Among BD patients, older age was associated increased accuracy on the ANMT (r=.52, p=.001) and the Chicago PEAT (r=.53, p<.001).
Discussion
This study examined the interface between emotion processing and working memory in pediatric BD patients. Consistent with our first hypothesis and past studies, BD youth performed more poorly compared to controls when rating the intensity of both positive and negative emotional expressions (McClure et al., 2005; Rich et al., 2008b; Schenkel et al., 2007). Emotion identification deficits were seen in both BD I and II patients, and remained significant after controlling for mood state and comorbid ADHD. These findings suggest that problematic emotion processing is significant across BD subtypes and is not due to greater clinical dysfunction and/or psychiatric comorbidity. Moreover, findings from this study support theories that emotion identification impairments may be a reliable endophenotype for BD (Brotman et al., 2008b). Indeed, a number of investigations have documented significant emotion identification impairments among BD youth compared to HC and other pediatric clinical populations including ADHD, conduct disorder, and major depressive disorder (Guyer et al., 2007; McClure et al., 2005; Rich et al., 2008a).
Additionally, compared to HC, pediatric BD patients demonstrated significantly more difficulty remembering facial stimuli regardless of emotional valence, however, working memory deficits were the most pronounced with negative stimuli and were specific to BD I patients. In particular, BD I patients were less accurate and slower to respond when identifying angry facial expressions. Therefore, type I BD patients appear to have a more impaired pattern of social cognitive dysfunction, regardless of emotional valence, but one that is exacerbated in negative contexts. Consistent with this, previous investigations in BD youth have reported a negative bias when identifying and rating the intensity of neutral facial expressions, as well as greater cognitive impairment when placed in negative contexts (Rich et al., 2005) and when required to take another's perspective in negative social situations (Schenkel et al., 2008). Moreover, our results are consistent with findings from Dickstein et al. (2007) who reported reduced memory for negative emotional faces among pediatric BD youth independent of clinical state or ADHD comorbidity. Data also support and extend theories that BD I is a more severe and debilitating illness than BD II, with greater functional impairment and more severe cognitive and social-cognitive dysfunction (Simonsen et al., 2008; Schenkel et al., 2012).
Our findings are consistent with neuroimaging models of the pathophysiology of pediatric BD that implicate a ventrolateral prefrontal-striatal-amygdala circuit (Blumberg et al., 2003; Dickstein et al., 2007; Passarotti et al., 2010, 2011; Pavuluri et al., 2007; Rich et al., 2005; Womer, Kalmar, Wang, & Blumberg, 2009). Specifically, both positive and negative emotions may impact higher cortical centers associated with emotion processing, however, negative emotions appear to have an even greater effect, and suggest that higher cortical centers of cognitive and affective circuitry are not efficiently recruited under the stress of being exposed to negative emotions. Imaging data from our laboratory from a separate study using this task indicated that BD youth exhibited greater deployment of emotion and emotion-regulation circuitry, particularly for negative faces (anterior cingulate cortex, orbito-fronal cortex, amyglala) and reduced deployment of working memory circuitry (Passarotti et al., 2010). Interestingly, while there was an improvement in prefrontal regions such as the VLPFC, increased amygdala activation relative to HC in response to emotional stimuli remained even after pharmacotherapy treatment, suggesting that overactivity of emotion-processing centers may be an important trait-marker for pediatric BD. In addition, this study found that performance on the Chicago PEAT and ANMT were significantly correlated, which lends further support for theories that basic emotion processing and higher-level cognitive control are interrelated and likely bidirectional, and play a significant role in the social-cognitive impairments seen in pediatric BD patients (Passarotti et al., 2011).
This study has a number of important clinical implications. First, emotion identification impairments were present across BD subtypes and were evident for both positive and negative faces. As such, psychosocial interventions with pediatric BD patients should incorporate specific exercises in face processing and emotion identification as part of a comprehensive social skills program. Training programs that focus on attending to salient facial features, face recognition, and emotion identification have been successful at improving interpersonal skills among children with Autism Spectrum Disorders (Hopkins et al., 2007; Tanaka et al., 2010). Cognitive remediation programs, including computer-based cognitive enhancement, emotion processing, and social skills training are currently underway in our laboratory. Second, results indicate a need to modify use of “negative consequences” in behavioral modification strategies in psychosocial treatments with BD youth. The excessive reactivity and reduced top-down modulation seen among pediatric BD patients in the face of negative and/or frustrating situations warrants modified treatment programs that focus on emotion regulation ability, positive reframing, and exercises in the development of more effective social skills and cognitive control (Pavuluri et al., 2006; West et al., 2009). Pharmacotherapy interventions also hold promise for improving cognitive and emotion processing abilities among pediatric BD patients. Treatment with Lamotrigine monotherapy has been shown to enhance the functioning of affective (VMPFC, VLPFC) and cognitive (DLPFC) circuitry, as well as improve working and verbal memory abilities among BD youth (Passarotti et al., 2011; Pavuluri et al., 2010a, 2010b).
Interestingly, age was not significantly correlated with emotion identification ability among healthy subjects, but was for BD patients. This raises the possibility that illness state may interfere with the successful development of emotion processing abilities, and could be an indication of why earlier age of onset is associated with a more chronic and severe form of illness among bipolar patients (Cater et al., 2003; Craney and Geller, 2003). It also underscores the need for early diagnosis and intervention. Better working memory performance was associated with increased age in both the HC and BD groups. This is not surprising as the neurocircuitry involved in working memory does not become fully active until late adolescence/early adulthood (Jolles, Kleibeuker, Rombouts, & Crone, 2011) whereas emotion recognition develops much earlier and reaches adult levels of competency by middle childhood (Durand, Gallay, Seigneuric, Robichon, & Baudouin, 2007).
There are a number of limitations to the current investigation. First, findings should be interpreted with caution given the relatively small sample used in this investigation. Future studies should be conducted using larger groups of type I and II BD youth, as well as pediatric patients that do not meet full diagnostic criteria for mania such as those diagnosed with severe mood dysregulation (SMD) who are characterized by a hyper-aroused state that is non-episodic with chronic irritability (Leibenluft, Charney, Towbin, Bhangoo, & Pine, 2003). Interestingly, pediatric BD and SMD patients both demonstrate cognitive as well as emotion processing impairments, but show differing profiles of underlying pathophysiology (see Leibenluft, 2011 for a review). Additionally, this study used only unmedicated pediatric bipolar patients, and therefore, findings cannot be considered conclusive. However, utilizing an unmedicated sample did allow for careful control of the severity of manic symptoms resulting in a more homogenous sample of patients. Future studies should employ controlled medication clinical trials using longitudinal follow-up designs to better understand the potential influences of mood state, illness severity, and pharmacotherapy on social-cognitive functioning among different clinical phenotypes of pediatric BD.
Our central findings indicate general emotion identification deficits across pediatric BD subtypes. Working memory deficits were evident irrespective of emotional valence, however, they were the most pronounced with negative emotional stimuli, and were specific to BD I patients. Pediatric BD I patients, therefore, display a more widespread and severe profile of social-cognitive dysfunction compared to BD II pediatric patients.
Acknowledgments
This study is supported by NARSAD, K23 NIH RR018638 and Susan and Walter Berger Family Foundation
References
- Adolphs R. The social brain: Neural basis of social knowledge. Annual Review of Psychology. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Text Revision (DSM-IV-TR) 4th Edn APA; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Bearden CE, Thompson PM, Dutton RA, Frey BN, Peluso MA, Nicoletti M, Dierschke N, Hayashi KM, Klunder AD, Glahn DC, Brambilla P, Sassi RB, Mallinger AG, Soares JC. Three-dimensional mapping of hippocampal anatomy in unmedicated and lithium-treated patients with bipolar disorder. Neuropsychopharmacology. 2008;33:1229–1238. doi: 10.1038/sj.npp.1301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Glahn DC, Caetano S, Olvera RL, Fonseca M, Najt P, Hunter K, Pliszka SR, Soares JC. Evidence for disruption on prefrontal cortical functions in juvenile bipolar disorder. Bipolar Disorders. 2007;9:145–159. doi: 10.1111/j.1399-5618.2007.00453.x. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Andrés M, Whiteman R, Hongyuan Zhang J, Gore JC, Charney DC, Krystal JH, Peterson BS. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Archives of General Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Bora E, Yücel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of Affective Disorders. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Botvinick M. Probing the Neural Basis of Body Ownership. Science. 2004;305:782–783. doi: 10.1126/science.1101836. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Guyer AE, Lawson ES, Horsey SE, Rich BA, Dickstein DP, Pine DS, Leibenluft E. Facial Emotion Labeling Deficits in Children and Adolescents at Risk for Bipolar Disorder. American Journal of Psychiatry. 2008a;165:385–389. doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Skup M, Rich BA, Blair KS, Pine DS, Blair JR, Leibenluft E. Risk for Bipolar Disorder is Associated with Face-Processing Deficits across Emotions. Journal of the American Academy of Child & Adolescent Psychiatry. 2008b;47:1455–1461. doi: 10.1097/CHI.0b013e318188832e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter TD, Mundo E, Parikh SV, Kennedy JL. Early age at onset as a risk factor for poor outcome of bipolar disorder. Journal Affective Disorders. 2003;37:297–303. doi: 10.1016/s0022-3956(03)00052-9. [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder. Archives of General Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. Journal of Neuroscience. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craney JL, Geller B. A prepubertal and early adolescent bipolar disorder-I phenotype: review of phenomenology and longitudinal course. Bipolar Disorders. 2003;5:243–256. doi: 10.1034/j.1399-5618.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Rich BA, Roberson-Nay R, Berghorst L, Vinton D, Pine DS, Leibenluft E. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disorders. 2007;9:679–692. doi: 10.1111/j.1399-5618.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Treland JE, Snow J, McClure EB, Mehta MS, Towbin KE, Pine DS, Leibenluft E. Neuropsychological performance in pediatric bipolar disorder. Biological Psychiatry. 2004;55:32–39. doi: 10.1016/s0006-3223(03)00701-7. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Wozniak J, Wilens TE, Henin A, Seidmen LJ, Petty C, Fried R, Gross LM, Faraone SV, Biederman J. Neurocognitive impairment in unaffected siblings of youth with bipolar disorder. Psychological Medicine. 2009;39:1253–1263. doi: 10.1017/S0033291708004832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand K, Gallay M, Seigneuric A, Robichon F, Baudouin JY. The development of facial emotion recognition: the role of configural information. Journal of Experimental Child Psychology. 2007;97:14–27. doi: 10.1016/j.jecp.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Gur RC, Gur RE, Smailis J, Skolnick B. Facial emotion discrimination: I. Task construction and behavioral findings in normal subjects. Psychiatry Research. 1992;42:231–240. doi: 10.1016/0165-1781(92)90115-j. [DOI] [PubMed] [Google Scholar]
- Geller B, Warner K, Williams M, Zimerman B. Prepubertal and young adolescent bipolarity versus ADHD: Assessment and validity using the WASH-U-KSADS, CBCL, and TRF. Journal of Affective Disorders. 1998;51:93–100. doi: 10.1016/s0165-0327(98)00176-1. [DOI] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. Journal of Neuroscience Methods. 2002;115:137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Guyer AE, McClure EB, Adler AD, Brotman MA, Rich BA, Kimes AS, Leibenluft E. Specificity of facial expression labeling deficits in childhood psychopathology. Journal of Child Psychology and Psychiatry. 2007;48:863–871. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- Herbener ES, Hill SK, Marvin RW, Sweeney JA. Effects of antipsychotic treatment on emotion perception deficits in first-episode schizophrenia. American Journal of Psychiatry. 2005;162:1746–1748. doi: 10.1176/appi.ajp.162.9.1746. [DOI] [PubMed] [Google Scholar]
- Hopkins IM, Gower MW, Perez TA, Smith DS, Amthor FR, Wimsatt FC, Biasini FJ. Avatar assistant: improving social skills in students with an ASD through a computer-based intervention. Journal of Autism and Developmental Disorders. 2011;41:1543–1555. doi: 10.1007/s10803-011-1179-z. [DOI] [PubMed] [Google Scholar]
- Jolles DD, Kleibeuker SW, Rombouts SA, Crone EA. Developmental differences in prefrontal activation during working memory maintenance and manipulation for different memory loads. Developmental Science. 2011;14:713–724. doi: 10.1111/j.1467-7687.2010.01016.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal Of The American Academy Of Child And Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. American Journal of Psychiatry. 2011;168:129–142. doi: 10.1176/appi.ajp.2010.10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. American Journal of Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Rich BA. Pediatric bipolar disorder. Annual Review of Clinical Psychology. 2008;4:163–187. doi: 10.1146/annurev.clinpsy.4.022007.141216. [DOI] [PubMed] [Google Scholar]
- McClure EB, Treland JE, Snow J, Dickstein DP, Towbin KE, Charney DS, Pine DS, Leibenluft E. Memory and learning in pediatric bipolar disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44:461–469. doi: 10.1097/01.chi.0000156660.30953.91. [DOI] [PubMed] [Google Scholar]
- Nowicki S, Duke MN. Individual differences in the nonverbal communication of affect: The Diagnostic Analysis of Nonverbal Accuracy Scale. Journal of Nonverbal behavior. 1994;18:9–35. [Google Scholar]
- Packard MG, Cahill LL. Affective modulation of multiple memory systems. Current Opinion in Neurobiology. 2001;11:752–756. doi: 10.1016/s0959-4388(01)00280-x. [DOI] [PubMed] [Google Scholar]
- Passarotti AM, Pavuluri MN. Brain Functional Domains Inform Therapeutical Interventions in Attention-deficit/hyperactivity Disorder and Pediatric Bipolar Disorder. Expert Review of Neurotherapeutics. 2011;11:897–914. doi: 10.1586/ern.11.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention deficit hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:1064–1080. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology. 2011;216:485–499. doi: 10.1007/s00213-011-2243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder (2005). A review of the past 10 years. Journal of American Academy of Child and Adolescent Psychiatry. 44:846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Marlow O'Connor M, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biological Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Fitzgerald JM, Wegbreit E, Sweeney JA. Risperidone and divalproex differentially engage the fronto-striato-temporal circuitry in pediatric mania: a pharmacological functional magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;5:157–170. doi: 10.1016/j.jaac.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Mohammed T, Carbray JA, Sweeney JA. Enhanced working and verbal memory after lamotrigine treatment in pediatric bipolar disorder. Bipolar Disorders. 2010a;12:213–220. doi: 10.1111/j.1399-5618.2010.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Parnes, Fitzgerald, Sweeney A pharmacological functional magnetic resonance imaging study probing the interface of cognitive and emotional brain systems in pediatric bipolar disorder. Journal of Child and Adolescent Psychopharmacology. 2010b;20:395–406. doi: 10.1089/cap.2009.0105. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Schenkel LS, Aryal S, Harral EM, Hill SK, Herbener ES, Sweeney JA. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. American Journal of Psychiatry. 2006;163:286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, O'Connor MM, Harral EM, Sweeey JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Research. 2008;162:244–255. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Schenkel LS, Aryal S, Harral EM, Hill SK, Herbener ES, Sweeney JA. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. American Journal of Psychiatry. 2006;163:286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Freeman LN, Mokros HB. Children's depressive rating scale-revised. Psychopharmacology Bulletin. 1985;21:979–989. [Google Scholar]
- Rich BA, Fromm SJ, Berghorst LH, Dickstein DP, Brotman MA, Pine DS, Leibenluft E. Neural connectivity in children with bipolar disorder: impairment in the face emotion processing circuit. Journal of Child Psychology & Psychiatry. 2008a;49:88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Grimley ME, Schmajuk M, Blair KS, Blair RJ, Leibenluft E. Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Development and Psychopathology. 2008b;20:529–546. doi: 10.1017/S0954579408000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Holroyd T, Carver FW, Onelio LM, Mendoza JK, Cornwell BR, Fox NA, Pine DS, Coppola R, Leibenluft E. A preliminary study of the neural mechanisms of frustration in pediatric bipolar disorder using magnetoencephalography. Depression and Anxiety. 2010;27:276–286. doi: 10.1002/da.20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Schmajuk M, Perez-Edgar KE, Pine DS, Fox NA, Leibenluft E. The impact of reward, punishment, and frustration on attention in pediatric bipolar disorder. Biological Psychiatry. 2005;58:532–539. doi: 10.1016/j.biopsych.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proceeding of the National Academy of Science. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel LS, Pavuluri MN, Herbener ES, Harral EM, Sweeney JA. Facial emotion processing in acutely ill and euthymic patients with pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1070–1079. doi: 10.1097/chi.0b013e3180600fd6. [DOI] [PubMed] [Google Scholar]
- Schenkel LS, West AE, Harral EM, Patel NB, Pavuluri MN. Parent–child interactions in pediatric bipolar disorder. Journal of Clinical Psychology. 2008;64:422–437. doi: 10.1002/jclp.20470. [DOI] [PubMed] [Google Scholar]
- Schenkel LS, West AE, Jacobs R, Sweeney JA, Pavuluri MN. Cognitive dysfunction is worse among pediatric patients with bipolar disorder type I than type II. Journal of Child Psychology and Psychiatry. doi: 10.1111/j.1469-7610.2011.02519.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C, Vaskinn A, Birkenaes AB, Engh JA, Hansen CF, JÓsdÓttir H, Ringen PA, Opjordsmoen S, Friis S, Andreassen OA. Neurocognitive profiles in bipolar I and bipolar II disorders: Differences in pattern and magnitude of dysfunction. Bipolar Disorders. 2008;10:245–255. doi: 10.1111/j.1399-5618.2007.00492.x. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Wolf JM, Klaiman C, Koenig K, Cockburn J, Herlihy L, Brown C, Stahl S, Kaiser MD, Schultz RT. Using computerized games to teach face recognition skills to children with autism spectrum disorder: the Let's Face It! program. Journal of Child Psychology and Psychiatry. 2010;51:944–952. doi: 10.1111/j.1469-7610.2010.02258.x. [DOI] [PubMed] [Google Scholar]
- Völlm B, Taylor A, Richardson P, Corcoran R, Stirling J, McKie S, Elliott R. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29:90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Wechsler D, Wycherley RJ, Benjamin L. Wechsler Memory Scale: WMS-III. Manual. The Psychological Corporation; San Antonio, TX: 1998. [Google Scholar]
- Weissman D, Perkins A, Woldorff M. Cognitive control in social situations: a role for the dorsolateral prefrontal cortex. Neuroimage. 2008;40:955–962. doi: 10.1016/j.neuroimage.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AE, Jacobs RH, Westerholm R, Lee A, Carbray J, Heidenreich J, Pavuluri MN. Child and family-focused cognitive-behavioral therapy for pediatric bipolar disorder: pilot study of group treatment format. Journal of the Canadian Academy of Child and Adolescent Psychiatry. 2009;18:239–246. [PMC free article] [PubMed] [Google Scholar]
- Womer FY, Kalmar KH, Wang F, Blumberg HP. A ventral prefrontal amygdala neural system in bipolar disorder: A view from neuroimaging research. Acta Neuropsychiatrica. 2009;21:228–238. [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegeler VE, Mayer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]