Abstract
This longitudinal cohort study evaluated the diversity, commonality, and stability of Streptococcus mutans genotypes associated with dental caries history. Sixty-seven 5 and 6 yr-old children, considered being at high caries risk, had plaque collected from baseline through 36 months for S. mutans isolation and genotyping with repetitive extragenic palindromic-PCR (4,392 total isolates). Decayed, missing, filled surfaces (dmfs/DMFS) for each child were recorded at baseline. At baseline, 18 distinct genotypes were found among 911 S. mutans isolates from 67 children (diversity) and 13 genotypes were shared by at least 2 children (commonality). The number of genotypes per individual was positively associated with the proportion of decayed surfaces (p-ds) at baseline. Twenty-four of the 39 children who were available at follow-up visits maintained a predominant genotype for the follow-up periods (stability) and was negatively associated with p-ds. The observed diversity, commonality, and stability of S. mutans genotypes represent a pattern of dental caries epidemiology in this high caries risk community, which suggest fewer decayed surfaces are significantly associated with lower diversity and stability of S. mutans genotypes.
Keywords: Streptococcus mutans, genotype, Genetic Diversity, Dental Caries
The advent of new molecular tools over the past few decades has substantially changed the understanding of dental caries microbial pathogenicity relative to bacterial detection and genotyping. Additionally, the concepts of dental caries etiology now focus on physical and host behavioral explanations relating this disease to a microbial ecological shift which is based on a physiologic imbalance between tooth mineral and biofilm (1, 2). Thus, among the concept involving the multifactorial nature of caries etiology, the resident microflora is considered to be interacting with teeth (host) and sugar (substrates) in an opportunistic relationship. Although dental caries is not a classical infectious disease (i.e., infections does not always result in disease), the infectious and transmissible characteristics of endogenous oral microflora (3–5) are considered important. Among oral bacteria, the mutans streptococci (MS, i.e., Streptococcus mutans and Streptococcus sobrinus) have been identified as the primary etiological agent associated with the initiation of dental caries in humans (4, 6, 7). Most of the evidence for MS association to dental caries has focused on S. mutans. To understand the infectious nature of dental caries regarding transmission of S. mutans, it may be useful to study characteristics of S. mutans genotypes, in terms of the concepts of diversity, commonality and stability in the oral cavity of individuals. Diversity is the number of different S. mutans genotypes found within an individual. Commonality is the number S. mutans genotypes shared among individuals in the population. Stability is the persistence of S. mutans genotypes over time in an individual.
Interestingly, children usually carry fewer genotypes (diversity) than adults (8–11), demonstrating that additional strains are acquired as one ages (8, 12). However if children have early childhood caries (ECC) they tend to have a greater diversity of S. mutans genotypes than caries-free children (13–15), indicating that frequent sugar consumption may exert a strong selective pressure (16, 17) for colonization of additional S. mutans genotypes (11).
Among several genetic analyses tools available, repetitive extragenic palindromic-polymerase chain reaction (rep-PCR) has been introduced as an effective method for bacterial strain typing with high reproducibility and throughput. This approach is advantageous in longitudinal study of species and strain level discrimination of many eubacteria pathogens (18–20). Previous studies have reported that rep-PCR combined with DNA chip analysis demonstrated high reproducibility (21, 22), which have been used to improve the probability of detecting S. mutans genotypes from a finite number of isolates (10, 23). Consequently, rep-PCR can be utilized to catalogue large numbers of S. mutans genotypes for longitudinal studies and for global comparison of S. mutans diversity.
Numerous studies have reported S. mutans genotypes; however extensive epidemiological longitudinal genetic characterization has not been feasible with previous methods due to limitations such as lack of reproducibility. This study evaluates the clonal diversity, commonality, and stability of S. mutans associated with caries epidemiology from a cohort of high caries-risk children followed over a 36 month time period using rep-PCR. It is hypothesized that the characteristics of S. mutans genotype infection is associated with the prevalence of dental caries.
Materials and Methods
Population and information
Oral samples were collected along with dental examination and preventive dental care from 5 to 6 yr old children in an elementary school at baseline and repeated at 6-month follow-up intervals over 3 yr. This African American community in Perry County Alabama is considered at high caries-risk because the population is a predominantly low socioeconomic status with non-fluoridated water supply (24). Furthermore, dental care access is low because the county has no dental provider for children, requiring patients to travel at least 50 km for dental treatment. Parental consent and child’s assent was obtained for this University of Alabama at Birmingham (UAB) Institutional Review Board approved study.
Children received a dental prophylaxis, professional fluoride treatment (5% sodium fluoride varnish), and personalized oral hygiene guidance upon dental examination and sample collection at follow-up visits. Assistance was provided for parents to seek regular dental care for the participants, especially if dental treatment needs were obvious. Oral observations and suggested treatment options were provided to the parents in writing following each examination. Oral examination by three trained and calibrated examiners (i.e., comparison of results of examinations of a sub-group of children examined by all three examiners) was performed using light source, compressed air source, mirror and explorer (no radiographs were taken). Decayed, missing, filled teeth/surfaces score, i.e., dmft (primary teeth)/DMFT (permanent teeth)/ and dmfs /DMFS, were recorded according to WHO criteria (25).
Oral sample collection
This study was designed to focus on individual newly erupting permanent molar teeth. Our objective was, not only to evaluate S. mutans genotype characteristics, but also how these characteristics relate to colonization of newly erupting teeth. In this regard, plaque was collected from primary molar teeth prior to newly erupting molars, thus the focus on molars. It is acknowledged that saliva is a more general surrogate for the bacterial community of the oral cavity; however the study design had the potential to provide data demonstrating more diversity between teeth within an individual. A sterile toothpick was used to collect a pooled plaque sample from mesial, distal, buccal, lingual, and occlusal surfaces of primary second molar teeth and any permanent molars that were present at the baseline visit. Additional plaque samples were collected at each 6-month follow-up visit, including up to 2 additional individually sampled erupting permanent molar teeth. Plaque samples were transferred to 1 mL of sterile reduced transport fluid (26). Although the focus of this study was plaque, whole saliva was also collected and served as a substitute when plaque S. mutans was not recovered (i.e., if plaque S. mutans was isolated at baseline but not at follow-up visits). Whole saliva was collected by chewing paraffin and expectorating approximately 5 ml into sterile 50 ml tubes. Samples were stored with ice and transported to the laboratory at UAB for processing within 24 h.
Isolation of mutans streptococci
Plaque samples were mixed and sonicated for 30 s on ice (Amplitude = 50; Vibra Cell, Sonics & Materials, Newtown, CT, USA). Following dilution using 0.05 M potassium phosphate buffer, each sample was plated in duplicate onto Mitis Salivarius agar (MS agar, Difco/Becton Dickinson, Sparks, MD, USA) supplemented with 20% sucrose and 200 unit/liter of Bacitracin (MSB) (27) with a Spiral Plater (Spiral System, Cincinnati, OH, USA) and incubated anaerobically (80% N2, 10% CO2 and 10% H2) at 37°C for 2 d (28). Mutans streptococci isolates were identified by colony morphology (and confirmed with PCR, see below) and counted on MSB media according the spiral plate instructions to calculate colony forming unit (CFU) per mL. Quartiles of CFU counts were compared to number of genotypes. No S. sobrinus was detected from any of the plaque isolates; therefore, the remainder of this study will focus on S. mutans. Seven to 10 isolated S. mutans colonies per sample (i.e., up to 3 different plaque samples per individual) were selected to provide a sufficient isolate number to determine genetic diversity as established, based on probability estimates (10). Colonies were inoculated into Todd-Hewitt broth and incubated anaerobically for 24–48 h (THB; BD, Sparks, MD, USA) before storage at −80°C in 20% glycerol. Frozen samples were pure streaked to TH agar and grown anaerobically for 48 h. Finally, individual isolated colonies were transferred into THB and incubated anaerobically for 18–24 h.
Extraction of DNA and rep-PCR
Cells obtained from the THB isolates were processed by methods previously described to extract DNA (23). Briefly, DNA was extracted with the Ultra Clean Microbial DNA Isolation Kit (MoBio Laboratories, Carlsbad, CA, USA). Quantitation and quality of DNA was assessed using a Nanodrop 1000 spectophotometer (Thermo Scientific, Wilmington, DE, USA). All isolates were confirmed as S. mutans prior to genotyping by means of SYBR Green PCR according to methods previously described using S. mutans specific primers (23, 29). Rep-PCR was performed with DiversiLab Streptococcus kit (bioMerieux, Durham, NC, USA) and ABI9700 thermocycler (Applied Biosystems, Foster City, CA, USA): initial denaturation for 2 min; 35 cycles of denaturation at 94°C for 30 s; annealing at 50°C for 30 s; extension at 70°C for 90 s; and a final extension at 70°C for 3 min (23).
DNA Chip application and DNA fingerprints analysis
The rep-PCR products were separated on microfluidics chips and an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) with the DNA Chip kit (bioMerieux) (21, 22). Virtual gel images of each strain were generated by DiversiLab v 3.3 software (bioMerieux). The genotype distinction criteria of DNA fingerprint was followed by the methods previously described (23). The generated reports were stored in DiversiLab’s online database for analysis and to construct a genotype library of S. mutans for future study.
Statistical analysis
Tables of CFU count quartiles vs. genotypes were condensed into 2 by 2 tables because of sparseness. Fisher’s exact tests were used to examine the association between CFU and number of genotypes. Kruskal-Wallis rank sum tests were conducted to evaluate the association between numbers of S. mutans genotypes and proportion of decayed surfaces from total surfaces of teeth (p-ds) at baseline sample collections. Generalized estimating equations using the negative binomial distribution were used to take into account the using multiple surfaces and visits per child to analyze the effect of S. mutans genotype stability on the occurrence of decayed surfaces. This analysis used children with data from at least 3 follow-ups. For each child included in the analysis, each visit was classified as either maintaining the most prevalent genotype as in the previous visit or not.
Results
Subject participation and dental examination
Ninety children initially matched the inclusion criteria of not having the first permanent molars erupted at baseline and 22/90 children were lost to follow-up and withdrawn. From this cohort, at baseline, 67 of the subjects had at least 7 isolates of S. mutans available for rep-PCR analysis. One child was failed to have enough S. mutans isolates at baseline, however the isolates were available from 18 month to 36 month. The 68 children consisted of 33 boys and 38 girls. Dental examination data (dmfs/DMFS) were recorded for the 67 children at baseline (mean dmfs = 12.9). When comparing dmfs scores between examiners on the same patients, there were no significant differences found (P = 0.60).
Diversity
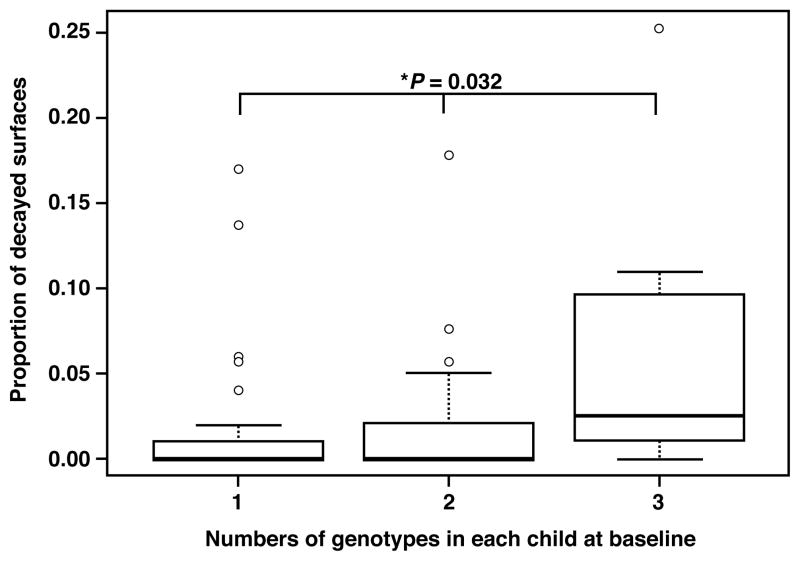
Overall 22 unique genotypes (G) were identified from a total of 4,392 isolates (mean 18 isolates/child/visit) collected from 67 children throughout the baseline and follow-up visits. These unique genotypes were use to define a S. mutans library (Table 1). All isolates were compared to this library to assign genotypes. The numbers of S. mutans genotypes identified per child (diversity) are shown in Table 2. At each visit, an average of 82% of children had less than 3 genotypes identified (mean genotypes/child = 1.7). The numbers of S. mutans genotypes were evaluated for association with CFU and demonstrated that the CFUs were not significantly correlated with the number of genotypes from this cohort (P = 0.25). The numbers of S. mutans genotypes were evaluated for association with caries examination data at baseline. Fig. 1 indicates that the numbers of genotypes per child were positively associated with proportion of decayed surfaces (i.e., decayed surfaces divided by the total number of tooth surfaces present, p-ds, P = 0.032).
Table 1.
Genotype catalog identified with number of children at given 6-month follow-ups: reflecting commonality and stability of S. mutans genotypes (total 4,392 isolates)
| Genotypes present at given follow-up | B | 6 M | 12 M | 18 M | 24 M | 36 M | |
|---|---|---|---|---|---|---|---|
| G01* |

|
13 | 8 | 10 | 14 | 9 | 10 |
| G02 | 1 | 0 | 0 | 0 | 1 | 0 | |
| G04 | 3 | 1 | 1 | 1 | 1 | 0 | |
| G05* | 1 | 1 | 3 | 2 | 2 | 2 | |
| G06* | 7 | 5 | 4 | 6 | 4 | 2 | |
| G07* | 8 | 4 | 3 | 5 | 8 | 3 | |
| G08 | 0 | 2 | 1 | 0 | 0 | 0 | |
| G09* | 11 | 7 | 9 | 8 | 6 | 3 | |
| G10** | 1 | 1 | 1 | 1 | 1 | 1 | |
| G11* | 3 | 3 | 3 | 3 | 2 | 2 | |
| G12* | 11 | 5 | 6 | 7 | 8 | 5 | |
| G13* | 7 | 8 | 7 | 4 | 5 | 4 | |
| G14* | 4 | 1 | 2 | 2 | 2 | 2 | |
| G15 | 2 | 0 | 0 | 1 | 1 | 1 | |
| G17 | 1 | 0 | 0 | 0 | 0 | 0 | |
| G18* | 12 | 8 | 7 | 9 | 8 | 7 | |
| G20 | 1 | 0 | 0 | 0 | 0 | 0 | |
| G22* | 9 | 6 | 6 | 6 | 5 | 5 | |
| G23* | 2 | 2 | 2 | 3 | 3 | 2 | |
| G25 | 0 | 0 | 0 | 1 | 0 | 0 | |
| G26 | 0 | 0 | 0 | 0 | 1 | 2 | |
| G27 | 0 | 1 | 1 | 0 | 0 | 0 | |
| Number of genotypes | 18 | 16 | 16 | 16 | 17 | 15 | |
| Number of isolates | 910 | 762 | 702 | 785 | 687 | 546 | |
| Number of children | 67 | 39 | 36 | 37 | 36 | 32 | |
| Number of children who have genotypes in common | 60 | 36 | 35 | 36 | 35 | 30 | |
B = baseline, M = months
genotypes present at all the follow-ups
genotype present with only one child
Table 2.
Number of children at given 6-month follow-ups: reflecting diversity of S. mutans genotypes (total 4,392 isolates)
| Number of genotypes/child | 1 | 2 | 3 | >3 | Mean genotypes/ child |
|---|---|---|---|---|---|
| B | 42 (63)* | 20 (30) | 5 (7) | 0 | 1.4 |
| 6 M | 25 (64) | 9 (23) | 2 (5) | 3 (8) | 1.6 |
| 12 M | 17 (47) | 11 (31) | 6 (17) | 2 (5) | 1.8 |
| 18 M | 16 (43) | 10 (27) | 8 (22) | 3 (8) | 2.0 |
| 24 M | 16 (44) | 13 (36) | 4 (11) | 3 (8) | 1.9 |
| 36 M | 19 (58) | 10 (30) | 4 (12) | 0 | 1.5 |
B = baseline, M = months,
Number (%) of children with a given number of S. mutans genotypes
Figure 1.
Box plot of S. mutans genotype diversity and caries activity. Number of S. mutans genotypes and caries activity at baseline was evaluated for 67 children. X axis: 1 = single genotype, 2 = 2 genotypes, >2 = more than 2 genotypes per individual at baseline. Y axis: Proportion of decayed surfaces (p-ds): total number of tooth surfaces present was included in the model as an offset variable. * Kruskal-Wallis rank sum test: χ2 = 6.8991, df = 2, P = 0.032.
Commonality
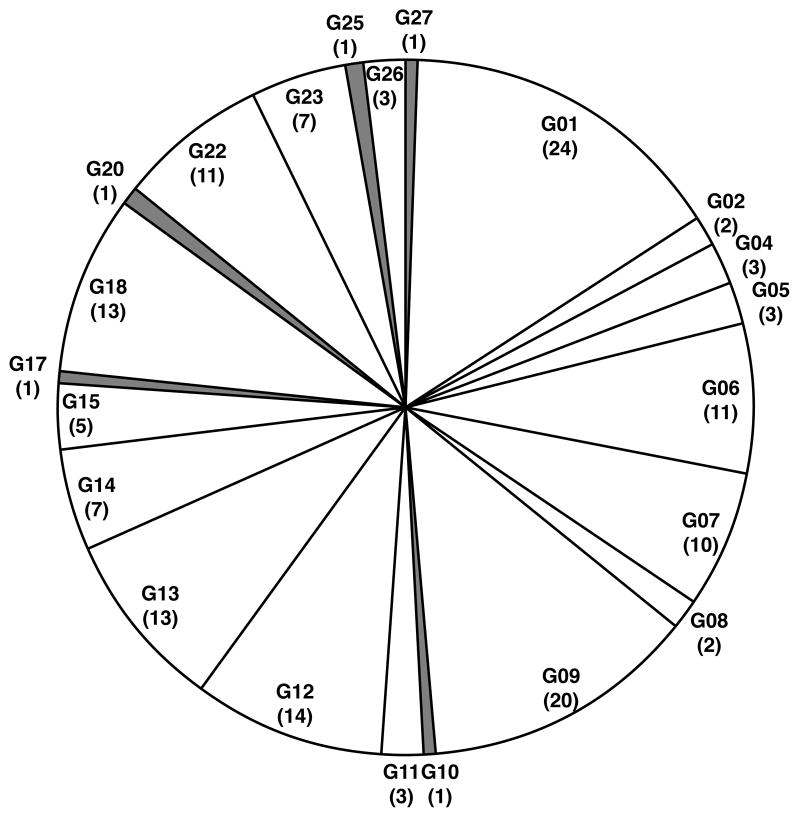
The commonality of genotypes between individuals was evaluated at each visit during the 36 months of this study (Table 1 and Fig. 2). Eighteen S. mutans genotypes were identified at baseline (4 additional genotypes at later visits) and 13 of the 18 genotypes were shared among the children. These 13 genotypes accounted for 96% of all the 4,392 isolates throughout the 36 months of this study. Further, 62 of 67 children (92.5%) shared at least one genotype with another child. The most prevalent genotype (G1) was observed in 24 of 67 (36%) children and made up 16% of all S. mutans isolated over 36 months.
Figure 2.
Pie chart of distribution of S. mutans genotypes over 36 months. Distribution and commonality is illustrated for the cohort of 67 children at baseline and during 36 month follow-up analysis. The genotype number is given with the number of individuals that share genotypes in parenthesis (i.e., if at least 2 children share a genotype). The genotypes which were not shared with anyone were indicated as grey shade (G10, 17, 20, 25, 27: less than 4% of total isolates).
Stability
Of the 67 children, 39 had at least 3 periods of follow-ups visits with at least 7 S. mutans isolates in each period available for stability analysis. The distribution of genotypes for each of the 39 subjects from each follow-up is summarized in Table 3 (4,034 total isolates). There was minimal evidence of newly erupting molars associated with newly acquired S. mutans genotypes. In this regard, thirteen of the original 18 baseline genotypes were found at each follow-up visit. Generally, when more than one genotype was found in an individual, one of the genotypes predominated (i.e., comprised more than half the isolates). Twenty-four of the 39 children (62%) maintained one predominant genotype throughout the 36 months. The presence of a predominant genotype was associated with lower p-ds (P < 0.0001) at baseline examination. Conversely, the 15 children that experienced changes in their predominant genotypes (associated also with multiple acquisitions or losses of genotypes, up to 5) during the follow-up visits were positively associated with higher p-ds (P < 0.0001).
Table 3.
S. mutans genotypes catalogued from 39 children (4,034 isolates) with isolates from at least 3 follow-ups
| Child ID# | B | 6M | 12M | 18M | 24M | 36M |
|---|---|---|---|---|---|---|
| 101 | G11 | G11 | G11 | G11 | G11 | G11,G26 |
| 102 | ND | ND | ND | G01,G15 | G01 | G01 |
| 104 | G01 | G01 | G01 | G01 | ND | ND |
| 106 | G07,G22 | G07 | G07 | G07 | G07,G15 | ND |
| 107* | G09,G22 | G09,G22 | G09,G22 | G09,G22 | G09,G22 | G09,G22 |
| 117 | G22 | G22,G09 | G22,G09 | G22 | G22 | G22,G26 |
| 119 | G18 | G18 | G18 | G18 | G18 | G18 |
| 120* | G13 | G13 | G13,G27 | 0 | 0 | 0 |
| 126 | G01 | G01,G22 | G01 | G01 | G01,G7 | G01 |
| 128 | G13,G20 | G13 | G13 | G13 | G13 | G13 |
| 134 | G01 | G01,G13 | G01 | G01 | G01 | G01 |
| 148 | G07,G18 | G07,G18 | ND | G06,G07,G18 | G07,G18 | G01,G07,G18 |
| 149* | G04,G05,G22 | G01,G04,G05,G13,G22 | G04,G05,G14 | G04,G05,G22 | G04,G05 | G05 |
| 150* | G01,G22 | G01,G22 | G01,G22 | G01,G14,G22 | G01,G12,G22 | G01 |
| 151 | G01,G14 | G14 | G14 | G14 | G14 | G14 |
| 159 | G12 | G12 | G12 | G12 | G01,G12 | G12 |
| 170 | G01,G12 | G01 | G01,G12 | G01,G12 | G01,G06,G12 | G01,G14 |
| 173 | G10 | G10 | G10 | G10 | G10 | G10 |
| 182* | G12 | G09 | G09,G12 | G09,G12,G23 | G12,G23 | G12,G23 |
| 184 | G07 | ND | ND | G07 | G07,G09 | G07 |
| 185 | G18 | G08,G18 | ND | G09,G18 | G18 | G18 |
| 193* | G13,G18 | ND | G09,G13,G18 | G01,G06,G09,G18 | G01,G09,G18 | G01,G15,G18 |
| 196 | G06 | G06 | G06 | G06 | G06 | G06 |
| 199 | G06,G18 | G06,G18,G23 | G18 | G01,G06,G18 | G06,G18 | G06,G18 |
| 201 | G01 | G01 | G01 | G01 | G01 | G01 |
| 211* | G13,G23 | G08,G09,G13,G23 | G08,G13,G23 | G13,G23 | G13,G23 | G13,G23 |
| 214* | G01,G06,G07 | G01,G06,G07 | G01,G06 | G01,G06,G07 | G01,G06,G07,G14 | G01,G07,G14 |
| 218* | G06 | G06,G12,G13,G22 | G01,G06,G13,G22 | G06,G13,G18,G22,G23 | G13,G22 | G13,G22 |
| 222* | G18 | G18 | G01,G05,G18 | G01,G05,G18 | G05,G12,G18 | G05,G18 |
| 226* | G12,G22 | G12 | G22 | G12,G22 | G22,G23 | G22 |
| 227* | G11,G12,G22 | G11 | G09,G11,G12,G22,G23 | G09,G11,G12,G22 | G09,G11,G12,G13,G26 | G11,G12,G22 |
| 228 | G09,G12 | G12 | G05,G06,G12 | G12 | G12 | ND |
| 232 | G09 | G09 | G09,G18 | G09 | G09 | G09 |
| 234 | G09,G18 | G18 | G09,G18 | G13,G18 | G18 | G18 |
| 239* | G07,G13 | G13 | G07,G13 | G07,G09 | G07,G13 | 0 |
| 241 | G12 | G12 | G12 | 0 | G02,G12 | G12 |
| 247* | G09 | G09 | G07,G09,G11,G13 | G01,G09 | G09 | G01,G09 |
| 252 | G11 | G11 | G09,G11 | G01,G11,G23 | 0 | 0 |
| 255* | G09,G18 | G09,G18 | G18 | G01,G18 | G18 | ND |
B = baseline, M = months, Bold genotypes indicate the predominant genotype at given period
0 = lost follow-up, ND= not detectable=less than 7 isolates, or failed to detect S. mutans isolates at given period
indicates that child experienced a change in predominant genotypes during follow-ups
Discussion
The limited diversity of S. mutans observed in this study (mean genotypes were less than 2 per child, Table 2) is consistent with other reports (30–32). Additionally, other studies have demonstrated that S. mutans infections are established in the early stages of dentition development and remain stable for several years (30, 32, 33). However, the present study’s findings add the dimension of genotype stability, which was not addressed in previous reports. It was important for this community-based study to provide some benefit to participants, therefore preventive care (prophylaxis and fluoride treatment), toothbrushes, toothpaste, floss, and oral health counseling was provided at each six month follow-up visit. Although these measures potentially could confound the results (i.e., intervention effect), baseline caries and genotype data established a starting point for which we monitored S. mutans genotypes over the 36 months reported herein. Following baseline identification of 18 genotypes, only 4 additional genotypes were found in 8 different children during the 36-month follow-up, accounting for 1.4% of the total isolates analyzed (63/4,392). Therefore, the 18 initial genotypes identified, not only reflect the low diversity, but also stability in the 13 common baseline genotypes that made up 93% of the total isolates in this longitudinal study. In contrast to our findings, other studies using different methods for defining genotypes have shown that individual children harbor distinct genotypes (13, 34), which are often shared with their biological mothers (31, 35). This variance of genotype commonality could be technique related (i.e., differences in AP-PCR vs. rep-PCR or differences in how similarities are defined), or differences in the populations studied. It is unlikely that the later is the case but rather, more likely to be technique related differences in genotyping. In a recent study, rep-PCR has been verified by the more extensive sequence-based MLST analysis as a reliable screening tool for large numbers of S. mutans epidemiological study (36). Therefore rep-PCR combined with DNA chip analysis, compared to the more labor and cost intensive MLST analysis, provides rapid and reproducible data for large numbers of S, mutans isolates (4,392) analyzed in this study.
In addition to the evaluation of stability and diversity, the longitudinal design of this study was aimed at the evaluation of the genotypic clonality when new teeth (i.e., permanent molars) erupt. For this reason, the sample collections mainly focused on plaque, however whole saliva samples were also collected and substituted when there was insufficient S. mutans isolates from plaque samples (i.e.,. less than 7, 11% of the total 4,392 isolates were from saliva). The timing of the initiation of this study was prior to eruption of permanent first molar teeth and, the majority of these teeth were fully erupted by the 36-month visit. No notable trend was observed in this study to indicate increased diversity was related to the eruption of specific permanent molar teeth (data not shown). These observations support the assertion that the genotypes of S. mutans for an individual appear to be established before eruption of permanent molars and that the eruption of permanent molar teeth is not related to increased diversity of S. mutans genotypes.
This study found that the genetic diversity of S. mutans is positively associated with the prevalence of dental caries, which is in accordance with previous reports (13, 37, 38). In addition, the study findings suggest that the predominance and/or certain combinations of specific S. mutans genotypes were associated with caries observed in this cohort; however the patient population size related to this observation is not adequate to make definitive conclusions related to these observations. Further, it is interesting that from the 67 children, one child was found with a comparatively high variety of genotypes (i.e., up to 5 at one time period, total of 7) during this longitudinal study and had high caries prevalence at baseline (dmfs = 36). Nonetheless, these interesting findings will require more observations and comparisons of S. mutans genotype profiles of children including related family members to observe vertical and horizontal commonality features associated with high caries susceptibility.
Future studies include evaluation of transmission using molecular typing methods in collaboration with rep-PCR. Vertical (maternal) transmission is generally accepted as the most common source of initial colonization (31, 39) with S. mutans, while other studies suggest as much as 31% paternal transmission (12). Additionally, significant horizontal transmission may take place between siblings and classmates/playmates (34, 40–42). Although evaluation of transmission is beyond the scope of this study, we observed that children, who were not biologically related (i.e., classmates in school), shared genotypes (commonality). At each follow-up period, an average of 77% of the genotypes was shared by at least one child. Future studies will evaluate evidence for vertical as well as horizontal transmission by longitudinal analysis of family members to supplement the school classmate data presented herein.
A reduced study population from the baseline (N = 67) to the 6 month follow-up (N = 39) was mainly due to the ability to retrieve S. mutans isolates from subsequent follow-up samples. Based on previous analysis reported for determination of minimal isolates required to provide representative sample of genotypes we required at least 7 isolates for each individual follow-up sample (10). Therefore children who had less than 7 isolates did not qualify for the longitudinal analyses; hence number of subsequent follow-up was reduced from 67 to 39.
It has been suggested that selective media such as MSB agar might limit the recovery rate of S. mutans (43, 44), however, this media has been successfully used and recommended for decades for isolating mutans streptococci (4, 27, 45). We have used this media extensively in the past and using it for this study provides continuity with our previous work. Furthermore, although S. sobrinus results were not included in this study, it was not excluded but rather rarely detected. The lack of S. sobrinus may be the result of the selective media used which may not be optimal for S. sobrinus. However, related quantitative-PCR studies of DNA extracted from whole plaque, saliva and tongue samples detected little to no S. sobrinus in the subjects of this large epidemiological study suggesting S. sobrinus may not be present in sufficient quantity to be detected by traditional culture methods in this population (data not shown).
Genotypic characterization of S. mutans using rep-PCR and DNA chip analyses provides practical and reproducible data to catalogue large numbers of S. mutans isolates longitudinally to establish a database for analysis of caries risk related factors. Thereby, this data provides an important foundation for tracking colonization with S. mutans in a high caries risk population. In this regard, this study is part of a larger study that will include investigation into S. mutans transmission among household family members (i.e., mother, father, siblings, and other cohabitating individuals such as aunts, uncles, cousins, and grandparents) of these children. The relative small number of genotypes among the thousands of isolates from this study established a S. mutans library that will facilitate comparisons of genotypes with other laboratories.
In conclusion, the automated rep-PCR DiversiLab system provides a reproducible, reliable, and cost effective genotyping screening tool for large numbers of S. mutans isolates. A limited number of genotypes (mean = 1.7) was identified within (diversity) and among (commonality) children, and was generally maintained within individuals for 36 months (stability). Less decayed surfaces were significantly associated with less diversity and stable S. mutans genotypes.
Acknowledgments
The authors appreciate the work of personnel contributing to this study: Ms. Stephanie McLean, Ms. Tonya Wiley, Dr. Steve Mitchell, Dr. Sonia Makhija, Dr. Rosalyn Bassett, Ms. Mary Slater, Ms. Frances Jackson, and the pediatric dental residents of the UAB School of Dentistry. This study was supported by NIH grant DE016684. Dr. Cheon was supported by the UAB Dr. Britta Rahemtulla Endowed fellowship and the DART32DE017601/T90DE022736 from the NIDCR.
Footnotes
Conflicts of Interest - The authors declare no conflict of interest.
References
- 1.Fejerskov O. Changing paradigms in concepts on dental caries: consequences for oral health care. Caries Res. 2004;38:182–191. doi: 10.1159/000077753. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi N, Nyvad B. Caries ecology revisited: microbial dynamics and the caries process. Caries Res. 2008;42:409–418. doi: 10.1159/000159604. [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz RJ. Acquisition and transmission of mutans streptococci. J Calif Dent Assoc. 2003;31:135–138. [PubMed] [Google Scholar]
- 4.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50 :353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seow WK. Biological mechanisms of early childhood caries. Community Dent Oral Epidemiol. 1998;26:8–27. doi: 10.1111/j.1600-0528.1998.tb02090.x. [DOI] [PubMed] [Google Scholar]
- 6.Tanzer JM, Livingston J, Thompson AM. The microbiology of primary dental caries in humans. J Dent Educ. 2001;65:1028–1037. [PubMed] [Google Scholar]
- 7.Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Soet JJ, Bokhout B, Buijs JF, van Loveren C, de Graaff J, Prahl-Andersen B. Transmission of mutans streptococci between mothers and children with cleft lip and/or palate. Cleft Palate Craniofac J. 1998;35:460–464. doi: 10.1597/1545-1569_1998_035_0460_tomsbm_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 9.Masuda N, Shimamoto T, Kitamura K, Sobue S, Hamada S. Transmission of Streptococcus mutans in some selected families. Microbios. 1985;44:223–232. [PubMed] [Google Scholar]
- 10.Cheon K, Moser SA, Whiddon J, Osgood RC, Momeni S, Ruby JD, Cutter GR, Allison DB, Childers NK. Genetic diversity of plaque mutans streptococci with rep-PCR. J Dent Res. 2011;90:331–335. doi: 10.1177/0022034510386375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alaluusua S, Matto J, Gronroos L, Innila S, Torkko H, Asikainen S, Jousimies- Somer H, Saarela M. Oral colonization by more than one clonal type of mutans streptococcus in children with nursing-bottle dental caries. Arch Oral Biol. 1996;41:167–173. doi: 10.1016/0003-9969(95)00111-5. [DOI] [PubMed] [Google Scholar]
- 12.Kozai K, Nakayama R, Tedjosasongko U, Kuwahara S, Suzuki J, Okada M, Nagasaka N. Intrafamilial distribution of mutans streptococci in Japanese families and possibility of father-to-child transmission. Microbiol Immunol. 1999;43:99–106. doi: 10.1111/j.1348-0421.1999.tb02380.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Q, Qin X, Qin M, Ge L. Genotypic diversity of Streptococcus mutans and Streptococcus sobrinus in 3–4-year-old children with severe caries or without caries. Int J Paediatr Dent. 2011;21:422–431. doi: 10.1111/j.1365-263X.2011.01145.x. [DOI] [PubMed] [Google Scholar]
- 14.Napimoga MH, Hofling JF, Klein MI, Kamiya RU, Goncalves RB. Tansmission, diversity and virulence factors of Sreptococcus mutans genotypes. J Oral Sci. 2005;47:59–64. doi: 10.2334/josnusd.47.59. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Q, Yu M, Min Z, Yi A, Chen D, Zhang Q. AP-PCR detection of Streptococcus mutans and Streptococcus sobrinus in caries-free and caries-active subjects. Mol Cell Biochem. 2012;365:159–164. doi: 10.1007/s11010-012-1255-5. [DOI] [PubMed] [Google Scholar]
- 16.Maciel SM, Marcenes W, Sheiham A. The relationship between sweetness preference, levels of salivary mutans streptococci and caries experience in Brazilian pre-school children. Int J Paediatr Dent. 2001;11:123–130. doi: 10.1046/j.1365-263x.2001.00259.x. [DOI] [PubMed] [Google Scholar]
- 17.Marshall TA, Broffitt B, Eichenberger-Gilmore J, Warren JJ, Cunningham MA, Levy SM. The roles of meal, snack, and daily total food and beverage exposures on caries experience in young children. J Public Health Dent. 2005;65:166–173. doi: 10.1111/j.1752-7325.2005.tb02807.x. [DOI] [PubMed] [Google Scholar]
- 18.Alam S, Brailsford SR, Whiley RA, Beighton D. PCR-Based methods for genotyping viridans group streptococci. J Clin Microbiol. 1999;37:2772–2776. doi: 10.1128/jcm.37.9.2772-2776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louws F, Rademaker J, de Bruijn F. The three Ds of PCR-based genomic analysis of phytobacteria: Diversity, Detection, and Disease Diagnosis. Annu Rev Phytopathol. 1999;37:81–125. doi: 10.1146/annurev.phyto.37.1.81. [DOI] [PubMed] [Google Scholar]
- 21.Bou G, Cervero G, Dominguez MA, Quereda C, Martinez-Beltran J. PCR-based DNA fingerprinting (REP-PCR, AP-PCR) and pulsed-field gel electrophoresis characterization of a nosocomial outbreak caused by imipenem- and meropenem-resistant Acinetobacter baumannii. Clin Microbiol Infect. 2000;6:635–643. doi: 10.1046/j.1469-0691.2000.00181.x. [DOI] [PubMed] [Google Scholar]
- 22.Healy M, Huong J, Bittner T, Lising M, Frye S, Raza S, Schrock R, Manry J, Renwick A, Nieto R, Woods C, Versalovic J, Lupski JR. Microbial DNA typing by automated repetitive-sequence-based PCR. J Clin Microbiol. 2005;43:199–207. doi: 10.1128/JCM.43.1.199-207.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moser SA, Mitchell SC, Ruby JD, Momeni S, Osgood RC, Whiddon J, Childers NK. Repetitive extragenic palindromic PCR for study of Streptococcus mutans diversity and transmission in human populations. J Clin Microbiol. 2010;48:599–602. doi: 10.1128/JCM.01828-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC. My Water’s Fluoride: Alabama. Centers for Disease Control and Prevention; 2008. [Accessed September 14, 2012]. [Google Scholar]
- 25.WHO. Oral health surveys: basic methods. 4. Geneva: World Health Organization; 1997. [Google Scholar]
- 26.Syed SA, Loesche WJ. Survival of human dental plaque flora in various transport media. Appl Microbiol. 1972;24:638–644. doi: 10.1128/am.24.4.638-644.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gold OG, Jordan HV, Van Houte J. A selective medium for Streptococcus mutans. Arch Oral Biol. 1973;18:1357–1364. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- 28.Lennette E, Spaulding E, Truant J. Manual of Clinical Microbiology. 2. American Society for Microbiology; Washington, D.C: 1974. [Google Scholar]
- 29.Yoshida A, Suzuki N, Nakano Y, Kawada M, Oho T, Koga T. Development of a 5′ nuclease-based real-time PCR assay for quantitative detection of cariogenic dental pathogens Streptococcus mutans and Streptococcus sobrinus. J Clin Microbiol. 2003;41:4438–4441. doi: 10.1128/JCM.41.9.4438-4441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emanuelsson IR, Thornqvist E. Genotypes of mutans streptococci tend to persist in their host for several years. Caries Res. 2000;34:133–139. doi: 10.1159/000016580. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Caufield PW. The fidelity of initial acquisition of mutans streptococci by infants from their mothers. J Dent Res. 1995;74:681–685. doi: 10.1177/00220345950740020901. [DOI] [PubMed] [Google Scholar]
- 32.Klein MI, Florio FM, Pereira AC, Hofling JF, Goncalves RB. Longitudinal study of transmission, diversity, and stability of Streptococcus mutans and Streptococcus sobrinus genotypes in Brazilian nursery children. J Clin Microbiol. 2004;42:4620–4626. doi: 10.1128/JCM.42.10.4620-4626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alaluusua S, Alaluusua SJ, Karjalainen J, Saarela M, Holttinen T, Kallio M, Holtta P, Torkko H, Relander P, Asikainen S. The demonstration by ribotyping of the stability of oral Streptococcus mutans infection over 5 to 7 years in children. Arch Oral Biol. 1994;39:467–471. doi: 10.1016/0003-9969(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Zou J, Shang R, Zhou XD. Genotypic diversity of Streptococcus mutans in 3- to 4-year-old Chinese nursery children suggests horizontal transmission. Arch Oral Biol. 2007;52:876–881. doi: 10.1016/j.archoralbio.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Ersin NK, Kocabas EH, Alpoz AR, Uzel A. Transmission of Streptococcus mutans in a group of Turkish families. Oral Microbiol Immunol. 2004;19:408–410. doi: 10.1111/j.1399-302x.2004.00163.x. [DOI] [PubMed] [Google Scholar]
- 36.Momeni SS, Whiddon J, Moser SA, Cheon K, Ruby JD, Childers NK. Comparative genotyping of Streptococcus mutans by repetitive extragenic palindromic polymerase chain reaction and multilocus sequence typing. Mol Oral Microbiol. 2013;28:18–27. doi: 10.1111/omi.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pieralisi FJ, Rodrigues MR, Segura VG, Maciel SM, Ferreira FB, Garcia JE, Poli-Frederico RC. Genotypic Diversity of Streptococcus mutans in Caries-Free and Caries-Active Preschool Children. Int J Dent. 2010:824–976. doi: 10.1155/2010/824976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Napimoga MH, Kamiya RU, Rosa RT, Rosa EA, Hofling JF, Mattos-Graner RO, Goncalves RB. Genotypic diversity and virulence traits of Streptococcus mutans in caries-free and caries-active individuals. J Med Microbiol. 2004;53:697–703. doi: 10.1099/jmm.0.05512-0. [DOI] [PubMed] [Google Scholar]
- 39.Douglass JM, Li Y, Tinanoff N. Association of mutans streptococci between caregivers and their children. Pediatr Dent. 2008;30:375–387. [PubMed] [Google Scholar]
- 40.Tedjosasongko U, Kozai K. Initial acquisition and transmission of mutans streptococci in children at day nursery. ASDC J Dent Child. 2002;69:284–288. 234–235. [PubMed] [Google Scholar]
- 41.Mattos-Graner RO, Li Y, Caufield PW, Duncan M, Smith DJ. Genotypic diversity of mutans streptococci in Brazilian nursery children suggests horizontal transmission. J Clin Microbiol. 2001;39:2313–2316. doi: 10.1128/JCM.39.6.2313-2316.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alves AC, Nogueira RD, Stipp RN, Pampolini F, Moraes AB, Gonçalves RB, Höfling JF, Li Y, Mattos-Graner RO. Prospective study of potential sources of Streptococcus mutans transmission in nursery school children. J Med Microbiol. 2009;58:476–481. doi: 10.1099/jmm.0.005777-0. [DOI] [PubMed] [Google Scholar]
- 43.Schaeken MJ, van der Hoeven JS, Franken HC. Comparative recovery of Streptococcus mutans on five isolation media, including a new simple selective medium. J Dent Res. 1986;65:906–908. doi: 10.1177/00220345860650060901. [DOI] [PubMed] [Google Scholar]
- 44.Wan AK, Seow WK, Walsh LJ, Bird PS. Comparison of five selective media for the growth and enumeration of Streptococcus mutans. Aust Dent J. 2002;47:21–26. doi: 10.1111/j.1834-7819.2002.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 45.Caufield PW, Cutter GR, Dasanayake AP. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J Dent Res. 1993;72:37–45. doi: 10.1177/00220345930720010501. [DOI] [PubMed] [Google Scholar]