Figure 2.
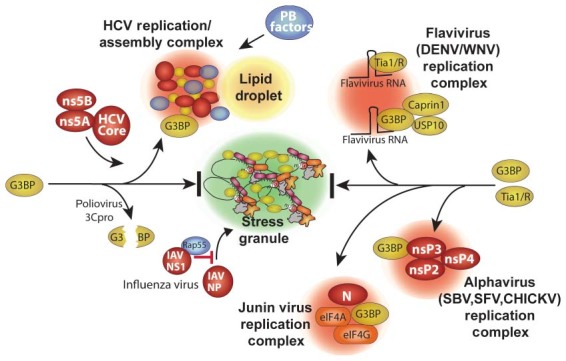
Virus blockade and co‐opting of stress granule responses. Specific points/proteins where viruses interact with and inhibit or divert the RNA granule assembly pathway are shown. Poliovirus 3Cproteinase cleaves the critical SG‐nucleating protein G3BP1. Several viruses co‐opt G3BP and divert it into novel virus‐induced foci. HCV diverts G3BP1 into replication/assembly complexes together with HCV core, ns5A and ns5B proteins that also associate with lipid droplets. HCVcomplexes also contain many PB components detailed in Figure 3. Flaviviruses divert G3BP1 (with USP10 and caprin1) and TIA1/TIAR to replication complexes by binding the host proteins on virus RNAs. Alphaviruses recruit G3BP1 into viral replication complexes via direct interaction viral protein nsP3. Junin virus (possibly N and G proteins) recruits G3BP1 into replication complexes that also contain translation factors eIF4G and eIF4A.
