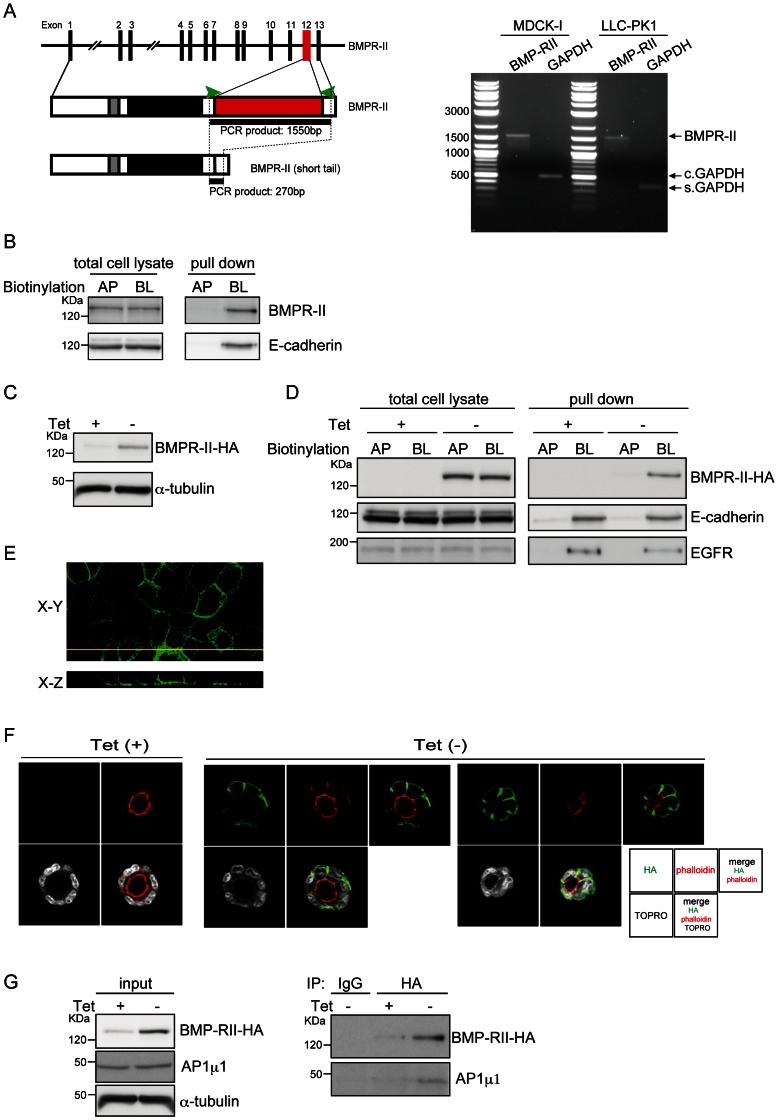
Figure 2. Basolateral localization of BMPR-II.
(A) A schematic illustration of the BMPR-II gene and protein, containing the transmembrane (grey) and kinase (black) domains and the region encoded by alternative exon 12 (red), is shown on the left. The primers used for PCR are shown as green arrowheads. Expression of alternative-splicing variants of BMPR-II in MDCK-I and LLC-PK1 cells was examined by RT-PCR. GAPDH was used as internal control for Canis lupus familiaris MDCK-I cells (c. GAPDH) and Sus scrofa LLC-PK1 cells (s. GAPDH). (B) After MDCK-I cells cultured on Transwells in a confluent condition were biotinylated from apical (AP) or basolateral (BL) sides, equal amounts of total proteins from each surface were subjected to SDS-PAGE (total cell lysate), or incubated with Streptavidin Sepharose 4B to isolate biotinylated proteins (pulldown). E-cadherin, a representative basolateral protein, was used as a control. (C) After withdrawal of tetracycline (Tet) from the culture media, MDCK-BR2 cells were lysed and subjected to immunoblot analysis with an antibody against HA. (D) MDCK-BR2 cells were seeded on Transwell plates to reach confluence in the presence or absence of tetracycline (Tet), and biotinylated from the apical (AP) or basolateral (BL) sides. Equal quantities of proteins were subjected to SDS-PAGE (total cell lysate), or incubated with Streptavidin Sepharose 4B to isolate biotinylated proteins, followed by SDS-PAGE (pulldown). E-cadherin and EGFR were used as representative basolateral proteins. (E) MDCK-BR2 cells were stained with an anti-HA antibody (green). XY (horizontal) and XZ (vertical) sections are shown in the top and bottom panels, respectively. (F) MDCK-BR2 cells cultured in Matrigel in the presence or absence of tetracycline (Tet) were stained with an anti-HA antibody (green), rhodamine-phalloidin (red), and TOPRO (white), followed by fluorescence imaging using a confocal laser scanning microscope. Micrographs of the two independent colonies were taken in the absence of Tet. (G) After MDCK-BR2 cells were cultured in the presence or absence of tetracycline (Tet), cell lysates were assayed for protein concentration and then subjected to immunoprecipitation. Co-purified AP1 µ1 was detected by immunoblotting with an anti-AP1 µ1 antibody. The expression levels of BMPR-II-HA, AP1 µ1, and α-tubulin in the same lysates were verified by immunoblotting.