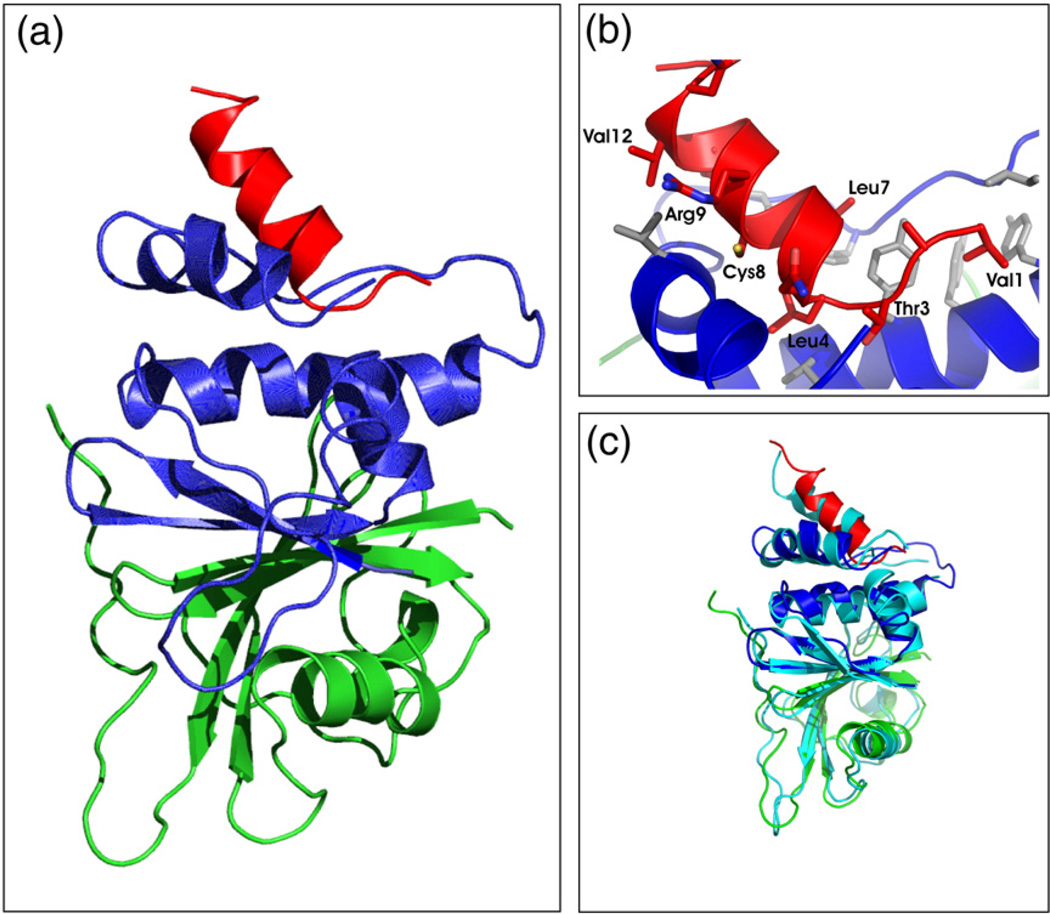
Fig. 5.
Tertiary structure of an SOCS3 SOCS box–elonginBC complex. (a) Ribbon diagram of the SOCS3 SOCS box (red) in complex with elonginC (blue) and elonginB (green). The interaction of the SOCS box occurs exclusively with elonginC and is mediated mostly by hydrophobic interactions. ElonginBC forms a tightly associated complex with the core of the association being a continuous β-sheet formed by residues from both proteins. A number of side-chain hydrophobic interactions further stabilise the complex. (b) Close view of the SOCS box elonginC interface with hydrophobic side chains from the SOCS box (red) labelled. The hydrophobic residues from elonginC (blue) that form the interface are shown in grey stick representation. (c) Ribbon diagram of the SOCS3 SOCS box in complex with elonginBC [same color scheme as in (a)] shown overlaid on the SOCS2/elonginBC structure (cyan).