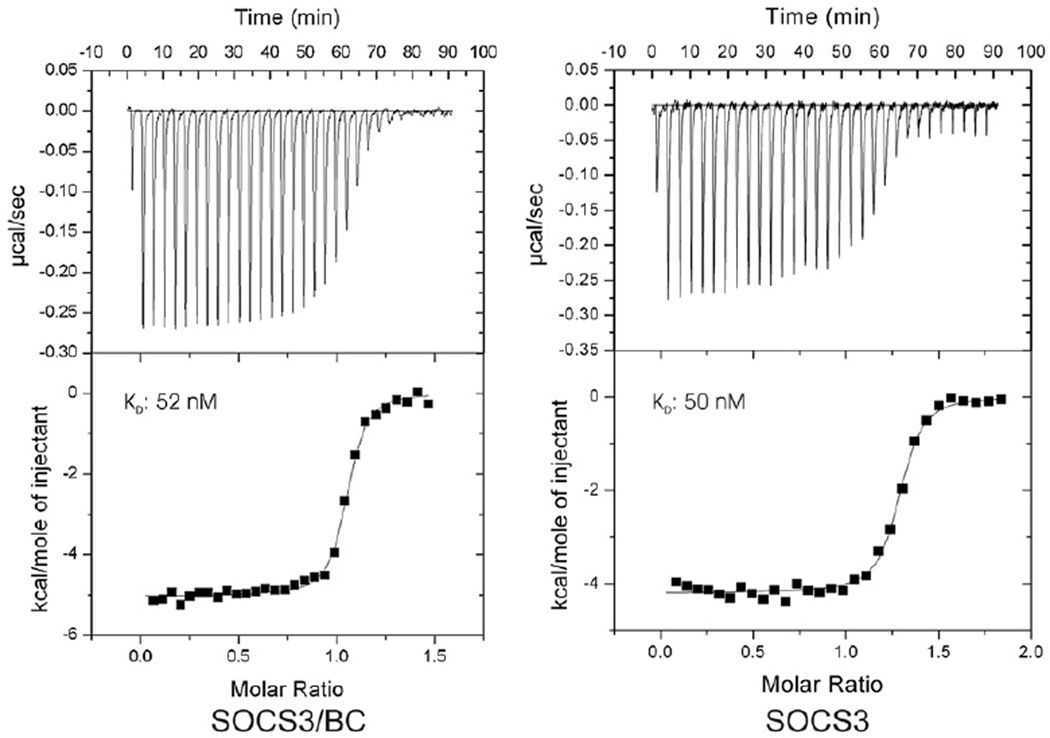
Fig. 7.
Isothermal titration analyses of the SOCS3–gp130 interaction in the presence and absence of elonginBC. ITC analyses show that a ternary SOCS–elonginBC complex is able to bind a tyrosine-phosphorylated peptide from the gp130 receptor with similar affinity to SOCS3 alone. Phosphopeptide (160 µM) was titrated into 20 µM SOCS3/elonginBC (left) or 20 µM SOCS3 (right) in 30×10-µL injections using a VP-ITC unit (Microcal). Both protein and peptide were prepared in Tris-buffered saline supplemented with 2 mM 2-mercaptoethanol. The titration curves fitted well to a single-site model with a Kd of 50±4 nM, ΔH −5030±30 kcal/mol, N 1.03 (SOCS3/BC) and Kd of 52±7 nM, ΔH −4200±30 kcal/mol, N 1.26. SOCS3, both as an isolated protein and in complex with elonginBC, lacked the PEST motif.