Abstract
In this study, the risk factors that may influence visual improvement after intravitreal ranibizumab (IVR) treatment for exudative age-related macular degeneration (AMD) were examined. From 2008 to 2012, 420 patients (448 eyes) with exudative AMD were prospectively registered at Seoul National University Hospital. From this group of patients, 125 eyes were included in this study. All patients were treated with 3 consecutive IVR injections. The visual acuity (VA) was evaluated at baseline and 1 month after the third ranibizumab injection. To evaluate the risk factors associated with VA improvement after IVR, patient demographic data and systemic risk factors were analyzed. Patients were divided into a poor VA improvement group and a good VA improvement group, with reference to the median visual improvement in all eyes. Among 125 eyes, 66 eyes (52.8%) were included in the responder group and 59 eyes (47.2%) in the non-responder group. The median VA improvement after 3 monthly ranibizumab injections was -0.05 logMAR. Multivariate analyses revealed that current smoking (adjusted OR, 7.540; 95% CI, 1.732-32.823) was independently associated with poor VA improvement after IVR treatment for exudative AMD. In conclusion, cigarette smoking is an independent risk factor for lower VA gains with IVR treatment for exudative AMD.
Keywords: Exudative Age-Related Macular Degeneration, Ranibizumab, Cigarette Smoking
INTRODUCTION
Exudative age-related macular degeneration (AMD) is a leading cause of visual loss in the elderly (1), and vascular endothelial growth factor (VEGF) is known to play a key role in the development of choroidal neovascularization (CNV) in the eyes with AMD (2). Intravitreal ranibizumab (IVR) injections result in the regression of CNV and improvements in visual acuity (VA) (3, 4). The majority of exudative AMD patients benefit from IVR, but the degree of vision recovery after IVR is variable (5-7). Several studies have suggested potential predictors of visual outcome. The suggested factors include baseline VA, CNV subtype, CNV lesion size, age at the time of ranibizumab treatment, and genetic polymorphism (3, 4, 7-9).
None of the factors that have been suggested to predict visual outcome can be modified by the treating physicians or by the patients. Identifying potential systemic or behavioral risk factors that can influence the visual outcome after anti-VEGF treatment in exudative AMD patients can be as important as the IVR treatment itself. Therefore, we conducted this investigation to identify modifiable systemic and behavioral risk factors that influence visual improvement after IVR in exudative AMD patients.
MATERIALS AND METHODS
The medical records for 448 eyes from 420 patients prospectively registered in an exudative AMD patient cohort between April 2008 and February 2012 at Seoul National University Hospital were retrospectively reviewed. Inclusion criteria for this study were as follows: 1) exudative AMD that was confirmed by physical examination, fluorescein angiography (FA), and optical coherence tomography (OCT) (Cirrus™; Carl Zeiss Meditec, Dublin, CA, USA), 2) 3 monthly intravitreal ranibizumab (Lucentis®; Novartis, Basel, Switzerland) injections, and 3) an OCT examination 1 month after the third injection. The exclusion criteria included the following: 1) poor compliance to treatment or loss from follow-up, 2) ranibizumab therapy in combination with any other AMD therapy (e.g., photodynamic therapy, intravitreal triamcinolone), 3) any ocular intervention within 3 months of the initial intravitreal ranibizumab injection, and 4) patient refusal to undergo blood sampling.
Hypertension, diabetes mellitus and smoking status, demographic data, and body type data (waist circumference, height, body weight, and body mass index [BMI]) were collected at baseline. Additionally, venous blood samples were drawn from all patients after 12 hr of fasting to examine systemic levels of C-reactive protein (CRP), total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglycerides. Smoking status was also recorded; a current smoker was defined as a patient who was an active smoker at enrollment. Non-smokers included both former smokers and patients who had never smoked.
Best-corrected VA was measured before treatment (pre-treatment) and 1 month after the third IVR injection (post-treatment). All VA measurements were converted to the logarithm of the minimal angle of resolution (logMAR) values for statistical analyses. The change in VA was calculated by subtracting the pretreatment logMAR VA from the post-treatment value. Based on the median VA change, patients were divided into 2 groups: those whose VA improved less than the median change (poor VA improvement group) and those whose VA improved equal or better than median change (good VA improvement group).
The CNV subtype was determined using findings from FA, spectral domain OCT and, if applicable, indocyanine green (ICG) angiography. Central macular thickness (CMT), as measured by OCT, was defined as the distance from the internal limiting membrane to Bruch's membrane at the fovea.
Statistical analyses were performed using SPSS software (Ver. 18, SPSS, Chicago, IL, USA). One-way analysis of variance (ANOVA), Student's t-tests, and Fisher's exact tests were performed on data, as applicable. Linear regression analysis and multivariate logistic regression analysis were also performed, adjusting for age, gender, diabetes mellitus and hypertension status, waist circumference, BMI, anticoagulant status, total cholesterol, and initial CMT.
Ethics statement
This study adhered to the tenets of the Declaration of Helsinki. The institutional review board of Seoul National University Hospital approved the study protocol (IRB number: H-1007-180-325). All participants provided informed consent before any study procedure was performed.
RESULTS
Among the 420 registered patients, 123 patients (125 eyes) who underwent blood sampling were included in this analysis. Fiftynine eyes (47.2%) were included in poor VA improvement group and 66 eyes (52.8%) were included in good VA improvement group. The mean and median VA changes after 3 monthly IVR injections were -0.15 ± 0.40 and -0.05 logMAR, respectively.
There were no significant differences in age, gender, waist circumference, height, body weight, BMI, CRP levels, total cholesterol or lipid (triglyceride, low-density lipoprotein, high-density lipoprotein) values, diabetes mellitus status, or hypertension status (Table 1). The proportions of patients taking anticoagulant drugs were also similar between the 2 groups. The only statistically different factor between the 2 groups was the patient's current smoking status (unadjusted odds ratio [OR], 4.81; 95% CI [confidence interval], 1.27-18.2). Eleven (18.6%) patients were current smokers among the 59 patients in the poor VA improvement group, in contrast to 4 (6.1%) among the 66 patients in the good VA improvement group (Fig. 1, 2).
Table 1.
Comparison of the poor and good VA improvement groups, divided by median visual acuity improvement (-0.05 log [minimum angle of resolution]).
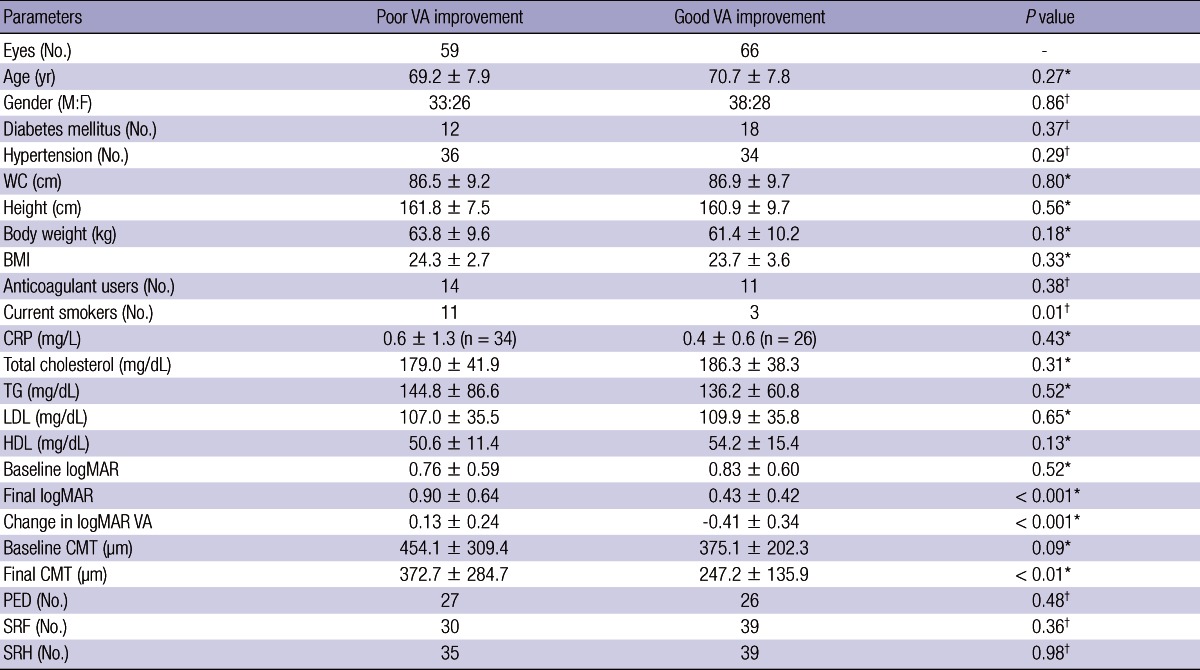
*Independent t-test; †Fisher's exact test. BMI, body mass index; CMT, central macular thickness; CRP, C-reactive protein; F, female; HDL, high-density lipoprotein; LDL, low-density lipoprotein; M, male; PED, pigment epithelium detachment; SRF, subretinal fluid; SRH, subretinal hemorrhage; VA, visual acuity; WC, waist circumference.
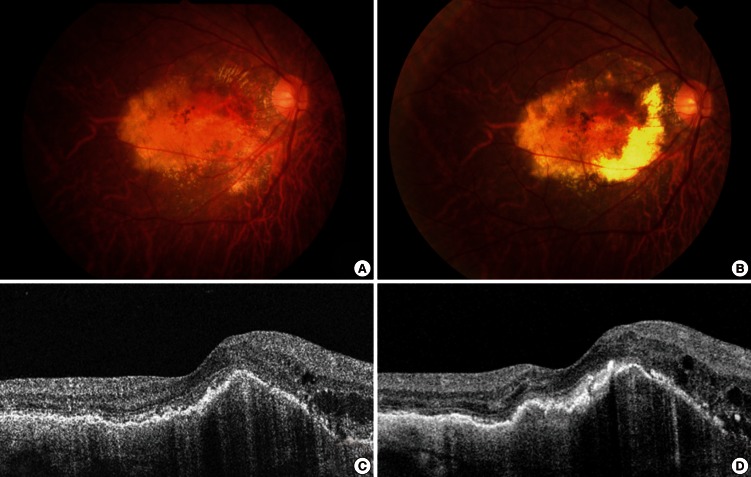
Fig. 1.
Fundus photographs and optical coherence tomography (OCT) images from a 57-yr-old male smoker. (A) A baseline fundus photograph shows submacular hemorrhage and exudate. (B) A post-treatment fundus photograph shows persistent submacular hemorrhage, extensive macular retinal pigment epithelial atrophy, and a disciform scar. Pre- (C) and post-treatment (D) OCT images show virtually no anatomical improvements following intravitreal ranibizumab treatment.
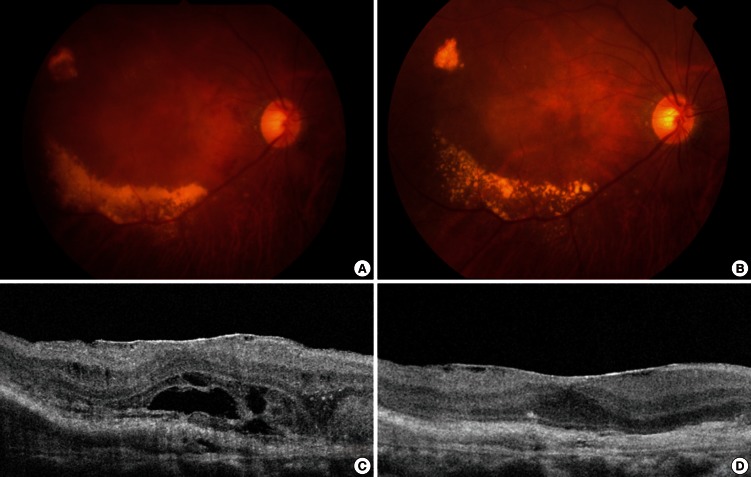
Fig. 2.
Fundus photographs and optical coherence tomography images (OCT) of a 73-yr-old male ex-smoker. (A) A baseline fundus photograph shows submacular hemorrhage, the choroidal neovascular membrane, and exudate. (B) A post-treatment fundus photograph shows resolution of the submacular hemorrhage, but choroidal neovascularization and exudate persists. (C) A baseline OCT image reveals subretinal fluid and a thickened choroidal neovascular membrane. (D) A post-treatment OCT image shows resolution of the previously observed subretinal fluid and thinning of a now inactive choroidal neovascular membrane.
Although the initial CMT was lower in the good VA improvement group than in the poor VA improvement group, the difference was not statistically significant (P = 0.09). However, the final CMT was significantly larger in the poor VA improvement group (P < 0.001). The incidences of pigment epithelial detachment, subretinal fluid accumulation, and subretinal hemorrhage were not significantly different between the 2 groups. The proportions of CNV subtypes were not significantly different between the 2 groups (data not shown).
Multivariate linear regression analysis after adjusting for age, gender, diabetes mellitus and hypertension status, waist circumference, BMI, anticoagulant status, and total cholesterol levels, showed that current smokers had a higher risk of a poor VA improvement (P = 0.004). In model 1, stepwise logistic regression revealed an independent association between current smoking (adjusted OR for age, gender, diabetes mellitus and hypertension status, waist circumference, BMI, anticoagulant status, and total cholesterol, 7.303; 95% CI, 1.698-31.414) and poor VA improvement (Table 2). In model 2, stepwise logistic regression suggested an independent association between current smoking (adjusted OR for age, gender, diabetes mellitus and hypertension status, waist circumference, BMI, anticoagulant status, total cholesterol, and baseline CMT, 7.540; 95% CI, 1.732-32.823) and poor VA improvement (Table 2).
Table 2.
Independent risk factors for poor visual acuity (VA) improvement after intravitreal ranibizumab treatment for exudative age-related macular degeneration
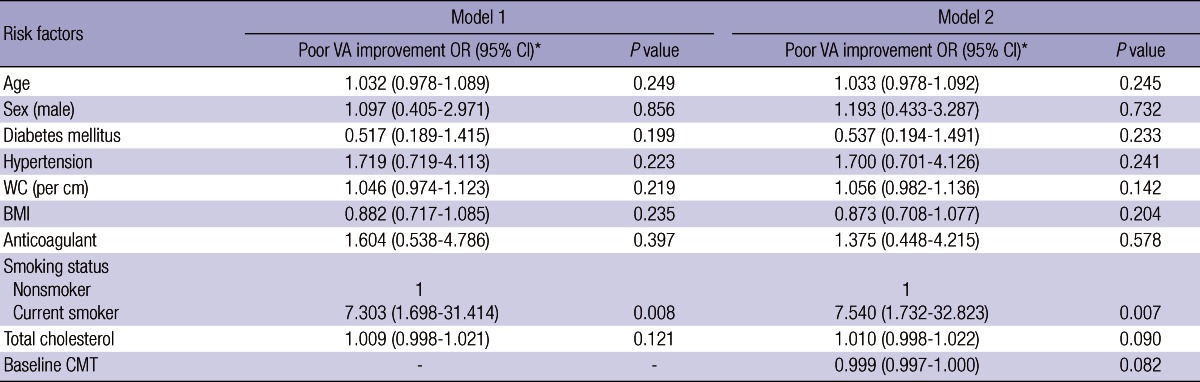
*Odds ratios and confidence intervals adjusted for other variables in the respective models by logistic regression analysis. AMD, age-related macular degeneration; BMI, body mass index; CI, confidence interval; CMT, central macular thickness; OR, odds ratio; WC, waist circumference.
DISCUSSION
Our data show that current cigarette smoking in exudative AMD patients is associated with a poor VA improvement following IVR therapy. Cigarette smoking increases the risk of developing AMD, with heavier smokers showing an increasingly greater risk of developing AMD (10-12). Additionally, a positive association between current and past smoking history and the development of exudative AMD has been reported (13). Fortunately, smoking cessation reduces the risk of developing AMD and of progression to neovascular AMD (12).
The mechanisms by which cigarette smoking negatively affects the retina are not well known. However, several hypotheses have been suggested. First, cigarette smoking is associated with increased oxidative stress, lipid peroxidation, fibrinogen levels, and platelet aggregation. It is also associated with reduced plasma high-density lipoprotein and antioxidant levels (11, 14, 15). Second, cigarette smoking can cause non-oxidative chemical damage to the retina. Nicotine promotes angiogenesis, both in vitro and in vivo, through nicotine-induced up-regulation of VEGF, a key molecule in the pathogenesis of neovascular AMD (16-19). Cigarette smoking also causes inflammation by activating complement C3 and other inflammatory mediators and reducing serum levels of complement factor H (18). Lastly, cigarette smoking can damage choroidal vessels and diminish choroidal blood flow through atherosclerosis and vasoconstriction, both of which are thought to play a role in AMD development (10, 20).
The effect of cigarette smoking on the response of exudative AMD to IVR treatment has not been widely studied. In our study, current smokers were more than 7 times more likely (adjusted OR, 7.540; 95% CI, 1.732-32.823) to have a poor VA improvement after IVR treatment than their non-smoking counterparts. In contrast, McKibbin et al. (7) reported that smoking status did not significantly affect VA change after IVR treatment, but patients who had never smoked tended to distinguish more letters in vision tests. These varying results may be the result of multiple compounding factors, such as genetic and/or racial differences.
In the present study, significant differences in CMT changes before and after therapy were not observed between current smokers and non-smokers. In addition, the baseline CMT values were not significantly different between the 2 groups. These results are consistent with those of previous reports that indicated that OCT findings are not sufficient to predict functional outcomes (21, 22).
The MARINA and ANCHOR studies showed that important predictors of VA outcomes are baseline VA, CNV lesion size, and age at the time of ranibizumab treatment (3, 4). Recent studies have also suggested an association between treatment response and genetic polymorphisms (7-9), which are, in most circumstances, not modifiable. In contrast, the present study suggests that an emphasis on smoking cessation before and even during treatment can improve the chances of a good VA response to IVR treatment.
The strength of our study is that it involved a prospective cohort study design, with all patients treated using a standardized protocol of 3 consecutive monthly injections and extensive analysis of numerous potential risk factors, including systemic and behavioral patterns among the exudative AMD patients.
The study, however, also has some limitations. The relatively small number of patients examined in this prospective study may have resulted in missed risk factors that might emerge from analyses of larger groups of patients. Additionally, smoking status was not quantified as pack-years, so we could not determine a smoking dose-response relationship. The relatively short 1-month follow-up period also did not allow long-term results to be examined. These limitations indicate that longer studies, involving larger groups of patients, are needed.
In conclusion, current cigarette smoking, a modifiable lifestyle choice, is an independent risk factor for a poor VA response to IVR used in the treatment of exudative AMD. Therefore, patients should be encouraged to stop smoking as early in their treatment course as possible.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997;81:154–162. doi: 10.1136/bjo.81.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR MARINA Study Group. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:246–252. doi: 10.1016/j.ophtha.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 4.Kaiser PK, Brown DM, Zhang K, Hudson HL, Holz FG, Shapiro H, Schneider S, Acharya NR. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144:850–857. doi: 10.1016/j.ajo.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, Puliafito CA, Davis JL, Flynn HW, Jr, Esquiabro M. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–583. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell P, Korobelnik JF, Lanzetta P, Holz FG, Prünte C, Schmidt-Erfurth U, Tano Y, Wolf S. Ranibizumab (Lucentis) in neovascular age-related macular degeneration: evidence from clinical trials. Br J Ophthalmol. 2010;94:2–13. doi: 10.1136/bjo.2009.159160. [DOI] [PubMed] [Google Scholar]
- 7.McKibbin M, Ali M, Bansal S, Baxter PD, West K, Williams G, Cassidy F, Inglehearn CF. CFH, VEGF and HTRA1 promoter genotype may influence the response to intravitreal ranibizumab therapy for neovascular age-related macular degeneration. Br J Ophthalmol. 2012;96:208–212. doi: 10.1136/bjo.2010.193680. [DOI] [PubMed] [Google Scholar]
- 8.Nischler C, Oberkofler H, Ortner C, Paikl D, Riha W, Lang N, Patsch W, Egger SF. Complement factor H Y402H gene polymorphism and response to intravitreal bevacizumab in exudative age-related macular degeneration. Acta Ophthalmol. 2011;89:e344–e349. doi: 10.1111/j.1755-3768.2010.02080.x. [DOI] [PubMed] [Google Scholar]
- 9.Wickremasinghe SS, Xie J, Lim J, Chauhan DS, Robman L, Richardson AJ, Hageman G, Baird PN, Guymer R. Variants in the APOE gene are associated with improved outcome after anti-VEGF treatment for neovascular AMD. Invest Ophthalmol Vis Sci. 2011;52:4072–4079. doi: 10.1167/iovs.10-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration: a case-control study in the agerelated eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology. 2000;107:2224–2232. doi: 10.1016/s0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seddon JM, Willett WC, Speizer FE, Hankinson SE. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA. 1996;276:1141–1146. [PubMed] [Google Scholar]
- 12.Khan JC, Thurlby DA, Shahid H, Clayton DG, Yates JR, Bradley M, Moore AT, Bird AC Genetic Factors in AMD Study. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol. 2006;90:75–80. doi: 10.1136/bjo.2005.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risk factors for neovascular age-related macular degeneration: the Eye Disease Case-Control Study Group. Arch Ophthalmol. 1992;110:1701–1708. doi: 10.1001/archopht.1992.01080240041025. [DOI] [PubMed] [Google Scholar]
- 14.Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006;124:995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- 15.Tomany SC, Wang JJ, Van Leeuwen R, Klein R, Mitchell P, Vingerling JR, Klein BE, Smith W, De Jong PT. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111:1280–1287. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Conklin BS, Zhao W, Zhong DS, Chen C. Nicotine and cotinine up-regulate vascular endothelial growth factor expression in endothelial cells. Am J Pathol. 2002;160:413–418. doi: 10.1016/S0002-9440(10)64859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, Tsao PS, Johnson FL, Cooke JP. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 18.Ni Dhubhghaill SS, Cahill MT, Campbell M, Cassidy L, Humphries MM, Humphries P. The pathophysiology of cigarette smoking and age-related macular degeneration. Adv Exp Med Biol. 2010;664:437–446. doi: 10.1007/978-1-4419-1399-9_50. [DOI] [PubMed] [Google Scholar]
- 19.Suñer IJ, Espinosa-Heidmann DG, Marin-Castano ME, Hernandez EP, Pereira-Simon S, Cousins SW. Nicotine increases size and severity of experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2004;45:311–317. doi: 10.1167/iovs.03-0733. [DOI] [PubMed] [Google Scholar]
- 20.Vingerling JR, Dielemans I, Bots ML, Hofman A, Grobbee DE, de Jong PT. Age-related macular degeneration is associated with atherosclerosis: the Rotterdam Study. Am J Epidemiol. 1995;142:404–409. doi: 10.1093/oxfordjournals.aje.a117648. [DOI] [PubMed] [Google Scholar]
- 21.Moutray T, Alarbi M, Mahon G, Stevenson M, Chakravarthy U. Relationships between clinical measures of visual function, fluorescein angiographic and optical coherence tomography features in patients with subfoveal choroidal neovascularisation. Br J Ophthalmol. 2008;92:361–364. doi: 10.1136/bjo.2007.123976. [DOI] [PubMed] [Google Scholar]
- 22.Unver YB, Yavuz GA, Bekiroglu N, Presti P, Li W, Sinclair SH. Relationships between clinical measures of visual function and anatomic changes associated with bevacizumab treatment for choroidal neovascularization in age-related macular degeneration. Eye (Lond) 2009;23:453–460. doi: 10.1038/eye.2008.349. [DOI] [PubMed] [Google Scholar]