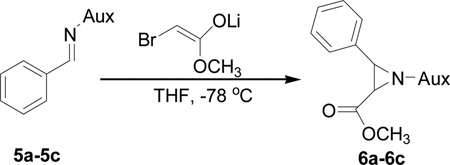
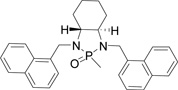
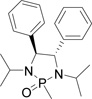
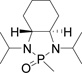
Table 1.
Optimizing the stereo effect of chiral N-phosphonyl iminesa
Reaction conditions: 0.57 mmol imine, 1.15 mmol methyl-2-bromo acetate, 1.20 mmol LiHMDS 14 mL solvent, −78 °C.
Diastereoselectivities were determined by 31P-NMR analysis of crude products.
>99:1 means only one isomer was observed by 31P NMR.