Abstract
The role of microtubule (MT) dysfunction in Parkinson's disease is emerging. It is still unknown whether it is a cause or a consequence of neurodegeneration. Our objective was to assess whether alterations of MT stability precede or follow axonal transport impairment and neurite degeneration in experimental parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in C57Bl mice. MPTP induced a time- and dose-dependent increase in fibres with altered mitochondria distribution, and early changes in cytoskeletal proteins and MT stability. Indeed, we observed significant increases in neuron-specific βIII tubulin and enrichment of deTyr tubulin in dopaminergic neurons. Finally, we showed that repeated daily administrations of the MT stabilizer Epothilone D rescued MT defects and attenuated nigrostriatal degeneration induced by MPTP. These data suggest that alteration of ΜΤs is an early event specifically associated with dopaminergic neuron degeneration. Pharmacological stabilization of MTs may be a viable strategy for the management of parkinsonism.
Axonogenesis and dendritogenesis, which are essential for the normal development of neurons, rely on the coordinated organization and dynamics of the actin and microtubule (MT) cytoskeleton. MTs are polymers built up by α/β tubulin heterodimers, characterized by an intrinsic resistance to bending and compression1. They are capable of switching between phases of slow growth and of rapid depolymerization2, features implicated in generating pushing and pulling forces within cells3. During neuronal differentiation, MTs are highly dynamic and ensure outgrowth and branching4. In mature neurons, MT stability increases5. The proper control of MT dynamics is essential for many neuronal activities, such as synaptic remodelling6, and both MT stability and neuronal functions are regulated through tubulin posttranslational modifications (PTMs)7.
Axon degeneration is a hallmark of neurodegenerative disorders and often precedes the onset of symptoms. Axonal destruction is an active process rather than a passive event8. Although little is known about the mechanisms involved, a growing body of evidence suggests a primary role of the MT system. In fact, MT fragmentation is the first detectable event during Wallerian degeneration9, and disorganized and bent MTs accompany the formation of retraction bulbs10 and axonal retraction11.
Parkinson's disease (PD) is the most common motor neurodegenerative disorder and each symptom depends on the reduction in dopamine (DA) levels in the striatum. Degeneration of nigrostriatal dopaminergic synaptic terminals precedes the death of dopaminergic neurons in the substantia nigra12. Many PD-linked proteins, such as α-synuclein13, parkin14, and leucine rich repeat kinase 215, modulate the stability of MTs, highlighting the crucial role of MTs during PD progression. Furthermore, PD is the only neurodegenerative disorder that is clearly related to environmental toxins, such as 1-methyl-4-phenylpiridinium (MPP+) or rotenone12, which both destabilize MTs16,17. Alterations in MT stability precede axonal transport impairment and neurite degeneration in MPP+-exposed PC12 cells18, suggesting that MT dysfunction plays an important role in mediating the toxicity of these compounds. It is noteworthy that both prophylactic and interventional treatments with the MT-stabilizer Epothilone D (EpoD), which is able to pass the blood-brain barrier, improve axonal MT density, reduce axonal dystrophy and alleviate cognitive deficits in transgenic mouse models of tauopathies19,20. It has also been shown that the dynamicity of MTs is increased in tau transgenic mice and that treatment with EpoD restores MT dynamics to baseline levels and exerts beneficial effects on behavior, tau pathology and neurodegeneration21.
We now show that C57Bl mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) express early alterations of α-tubulin PTMs specifically in dopaminergic neurons, and that systemic injections of EpoD rescue MT defects and attenuate nigrostriatal degeneration in mice with parkinsonian symptoms produced by exposure to the toxin MPTP.
Results
Treatment paradigm underlines early alterations
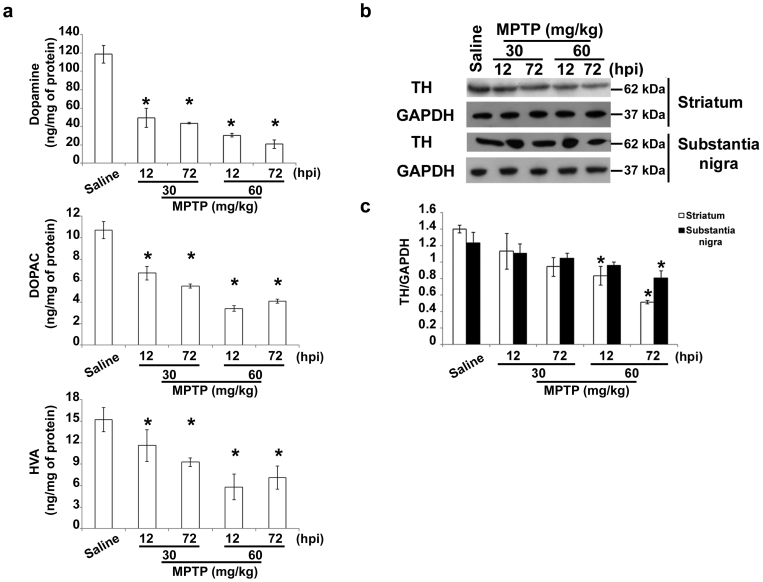
One single dose of MPTP (30 mg/kg, i.p.) induced about 50–60% reduction in striatal DA levels 7 days later in C57Bl mice, as expected22. To highlight very early alterations, mice were treated either with a single dose (30 mg/kg, i.p.) or with a cumulative dose (60 mg/kg) of MPTP, and sacrificed 12 or 72 h later. Biochemical analyses showed that MPTP induced a significant reduction in striatal DA and its metabolites, a sign of ongoing neurodegeneration (Fig. 1a). Western Blotting analysis showed that TH, the rate-limiting enzyme of DA biosynthesis, was decreased in the striatum and substantia nigra only with the highest dose of MPTP (Fig. 1b,c). Ara and colleagues23 showed that MPTP induces early inactivation of the enzyme, followed by reduction in TH levels; therefore, mice treated with the low dose of MPTP (30 mg/kg), which do not show any changes in TH levels, can be considered to be in an early phase of neurodegeneration, and may be used to uncover very early alterations in experimental parkinsonism.
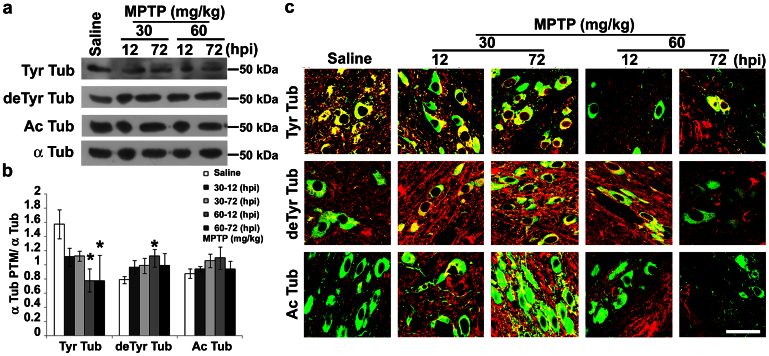
Figure 1. Treatment paradigm underlines early alterations.
(a) Biochemical analyses of striatal dopamine, DOPAC and HVA levels in C57Bl mice injected with saline or MPTP (30 mg/kg, i.p., single injection or 20 mg/kg, i.p., × 3, 2 h apart) and killed 12 or 72 hours later (mean ± S.E.M., n = 8–10 mice per group). *P < 0.05; one-way ANOVA, Dunnett post hoc versus saline-injected mice. (b) Immunoblot of TH levels in lysates of the corpus striatum and substantia nigra of mice injected with saline or MPTP as in a. (c) Densitometric analyses of immunoblot reported in b (mean ± S.E.M., n = 8–10 mice per group). *P < 0.05; one-way ANOVA, Dunnett post hoc test versus saline-injected mice. hpi = hours post last injection of MPTP.
MPTP causes axonal transport impairment in dopaminergic fibres
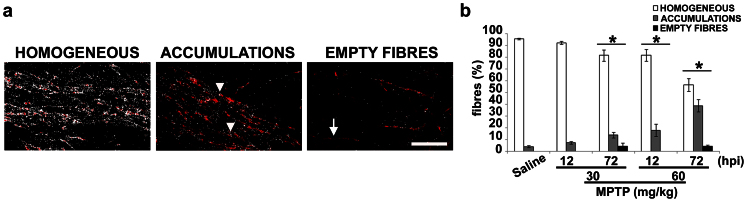
Mitochondria, as well the other organelles, accumulate into varicosities when axonal transport is impaired24, and there is evidence that MPP+ interferes with this process18,25,26. Therefore, to assess the status of axonal transport in MPTP-treated mice, we evaluated mitochondria distribution within dopaminergic fibres by performing a double immunohistochemical analysis in sagittal sections of TH and voltage-dependent anion channel (VDAC)-porin, a structural protein of the mitochondrial pore. We observed dopaminergic fibres with a homogeneous distribution of mitochondria and fibres showing sparse or accumulated mitochondria (Fig. 2a, arrowheads). It is noteworthy that only mice treated with MPTP and killed 72 h later showed empty fibres (Fig. 2a, arrow), as the mitochondria had been kept out of neuronal processes. Quantification of the different types of fibres (Fig. 2b), showed a time- and dose-dependent increase in fibres with altered mitochondria distribution in treated mice. No significant differences were observed between control mice and mice treated for 12 h with 30 mg/kg MPTP. These data show that MPTP affects axonal transport in vivo.
Figure 2. MPTP causes axonal transport impairment.
(a) Mask projections of sagittal sections of the nigrostriatal pathway in mice injected with saline or MPTP as in Fig. 1, showing the distribution of mitochondria (white spots) inside dopaminergic fibres (red). Arrowheads indicate mitochondria accumulations and arrow highlights an empty fibre. Scale bar = 20 μm. (b) Percentage of fibres displaying a homogeneous distribution of mitochondria, fibres showing dispersed or accumulated mitochondria or empty fibres. hpi = hours post last injection of MPTP (mean ± S.E.M., n = 9 sections from 3 different mice per group). *P < 0.05; χ2 test versus saline-injected mice.
MPTP increases the neuron-specific βIII tubulin isotype
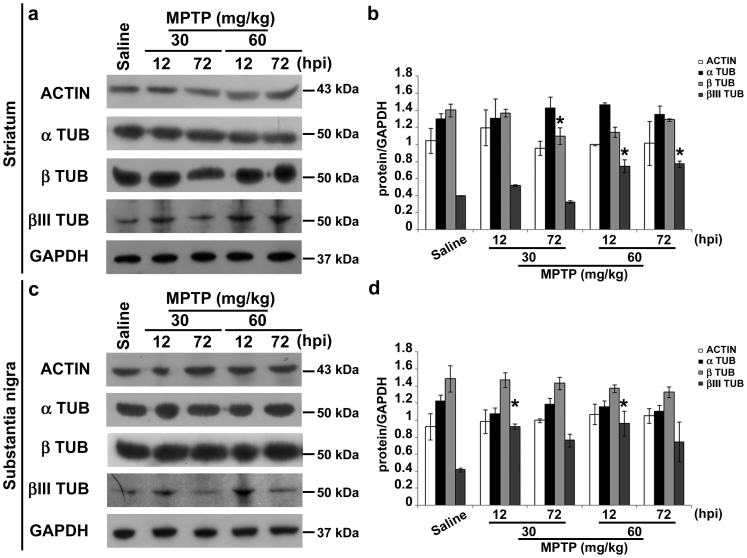
Axonal transport defects could be related to alterations of cytoskeletal proteins and molecular motors. Thus, we evaluated levels of actin, α- and β-tubulin, and the neuron-specific βIII tubulin isotype. Western blotting and densitometric analyses showed that levels of actin and α/β tubulin were unchanged in the striatum (Fig. 3a,b) and substantia nigra (Fig. 3c,d) of MPTP-treated mice. β-tubulin was significantly reduced in the striatum of mice treated with MPTP (30 mg/kg) and killed 72 h later (Fig. 3a,b). The specific change in β-tubulin is not surprising, because Chung and colleagues27 already showed differential variations in α- and β-tubulin monomers in a rat model of synucleinopathy, and we have recently found significant enrichment of β-tubulin in the fibroblasts of PD patients carrying parkin mutations28. On the other hand, the neuron-specific βIII tubulin isotype was significantly increased by MPTP treatment in both striatum and substantia nigra, although at different time points (Fig. 3). Levels of kinesin and dynein, responsible for MT-dependent anterograde and retrograde axonal transport, respectively29, were not affected by MPTP treatment (Supplementary Fig. S1), suggesting that axonal transport defects may be due to alternative factors.
Figure 3. MPTP treatment increases the βIII tubulin isotype.
(a) Immunoblots of actin, α-tubulin, β-tubulin and βIII tubulin in lysates of striatum of mice treated as in Fig. 1. (b) Densitometric analyses of immunoblot reported in a (mean ± S.E.M., n = 4–6 individuals per group). *P < 0.05; one-way ANOVA, Dunnett post hoc test versus saline-injected mice. (c) Immunoblot of actin, α-tubulin, β-tubulin and βIII tubulin in lysates of substantia nigra of mice treated as in Fig. 1. (d) Densitometric analyses of immunoblot reported in c (mean ± S.E.M., n = 4–6 individuals per group). *P < 0.05; one-way ANOVA, Dunnett post hoc test versus saline-injected mice. hpi = hours post last injection of MPTP.
MPTP specifically affects MT stability in dopaminergic neurons
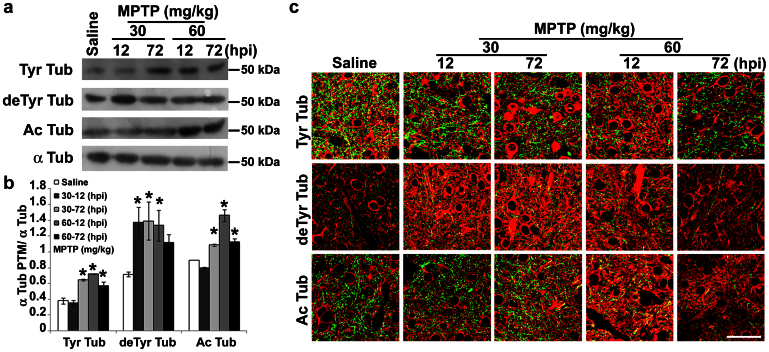
α-tubulin PTMs are usually used as markers of MTs with different stability, being tyrosinated (Tyr) MTs the most dynamic, and acetylated (Ac) or detyrosinated (deTyr) MTs the most stable pools7. Recently, it has been shown that α-tubulin PTMs are directly linked to neurodegeneration in mice30 and MPP+ affects α-tubulin PTMs in PC12 cells18. Therefore, we evaluated MT stability in the striatum (Fig. 4) and substantia nigra (Fig. 5) of MPTP-treated mice, as well as along their nigrostriatal pathway (Supplementary Fig. S2). Biochemical analyses showed the enrichment of deTyr tubulin in striatum (Fig. 4a,b), which was detectable already 12 h after the injection of the lowest dose of MPTP, and a later increase in both Tyr and Ac tubulins. Confocal analyses and the evaluation of Manders' coefficients (M1 and M2, reported in Table 1 and Supplementary Table S1, respectively), which are good indicators of relative signal distribution31, showed that changes in the levels of tubulin PTMs were located in dopaminergic terminals. In fact, we observed an increased co-localization between deTyr or Ac Tub and TH signals (Fig. 4c) and significant elevation in M1 parameter (Table 1). On the other hand, the significant decrease in the M1 parameter (Table 1) demonstrated that the enrichment of Tyr tubulin was associated with neurons residing in the striatum.
Figure 4. MPTP affects MT stability in dopaminergic terminals.
(a) Immunoblot of levels of tyrosinated tubulin (Tyr Tub), detyrosinated tubulin (deTyr Tub) and acetylated tubulin (Ac Tub) in lysates of striatum of mice treated as in Fig. 1. (b) Densitometric analyses of immunoblot reported in a (mean ± S.E.M., n = 4–6 mice per group). For the quantitation, values of each α-tubulin PTM were normalized on the level of α-tubulin (α Tub) of the relative sample. *P < 0.05; one-way ANOVA, Dunnett post hoc test versus saline-injected mice. (c) Confocal images of striatum of mice treated as in Fig. 1. Green represents TH staining and red signals the various tubulin PTMs. Scale bar = 50 μm. hpi = hours post last injection of MPTP.
Figure 5. MPTP affects MT stability in dopaminergic neurons.
(a) Immunoblot of levels of tyrosinated tubulin (Tyr Tub), detyrosinated tubulin (deTyr Tub) and acetylated tubulin (Ac Tub) in lysates of substantia nigra of mice treated as in Fig. 1. (b) Densitometric analyses of immunoblot reported in a (mean ± S.E.M., n = 4–6 mice per group). For the quantitation, values of each α-tubulin PTM were normalized on the level of α-tubulin (α Tub) of the relative sample. *P < 0.05; one-way ANOVA, Dunnett post hoc test versus saline-injected mice. (c) confocal images of substantia nigra of mice treated as in Fig. 1. Green represents TH staining and red signals the various tubulin PTMs. Scale bar = 50 μm. hpi = hours post last injection of MPTP.
Table 1. Analysis of M1 parameter (Tubulins vs. TH) in striatal sections.
| Tyr Tubulin | deTyr Tubulin | Ac Tubulin | |
|---|---|---|---|
| Saline | 0.41 ± 0.032 | 0.30 ± 0.025 | 0.16 ± 0.013 |
| MPTP, 30 mg/kg (12 h) | 0.27 ± 0.021 (*) | 0.39 ± 0.013 | 0.28 ± 0.028 (*) |
| MPTP, 30 mg/kg (72 h) | 0.27 ± 0.048 (*) | 0.51 ± 0.027 (*) | 0.33 ± 0.021 (*) |
| MPTP, 60 mg/kg (12 h) | 0.17 ± 0.029 (*) | 0.34 ± 0.034 | 0.36 ± 0.019 (*) |
| MPTP, 60 mg/kg (72 h) | 0.24 ± 0.043 (*) | 0.32 ± 0.030 | 0.16 ± 0.023 |
Data are expressed as mean ± S.E.M., n = 4 sections for each mouse from 4–6 mice per group. *P < 0.05; one-way ANOVA, Dunnett post hoc: versus saline-injected mice.
Western blotting analysis performed on substantia nigra lysates (Fig. 5a, b), revealed a significant decrease in Tyr tubulin in MPTP-treated mice, suggesting that the dynamic MT pool was reduced, as we already showed in cultured cells18. On the other hand, MPTP induced a significant enrichment of deTyr tubulin in mice treated with MPTP (60 mg/kg) and killed 12 h later, suggesting an increase in stable MTs, which mirrors the changes observed in the striatum. Confocal analyses showed that α-tubulin PTM changes occurred in dopaminergic cell soma (Fig. 5c), and the quantification of fluorescence intensity within single cells (Supplementary Table S2) showed that alterations of MT stability are higher in dopaminergic neurons. Indeed, we observed a significant reduction in Tyr tubulin and an early increase in deTyr and Ac tubulins that resulted in the decrease of stable MTs.
Finally, as α-tubulin PTMs influence axonal transport32,33, we evaluated potential changes within dopaminergic fibres by confocal microscopy (Supplementary Fig. S2). Besides the slight changes observed in Tyr and Ac tubulin content, the overall result we obtained is an early increase in deTyr tubulin, in line with the modifications of this subset of stable MTs in the striatum and substantia nigra of MPTP-treated mice, which could be responsible for the impairment of axonal transport, as already suggested18. Taken together, these results show that MPTP affects MT stability in vivo, and that the alteration of α-tubulin PTMs is an early event specifically associated with dopaminergic neurons.
Stabilization of MT attenuates MPTP-induced neurodegeneration
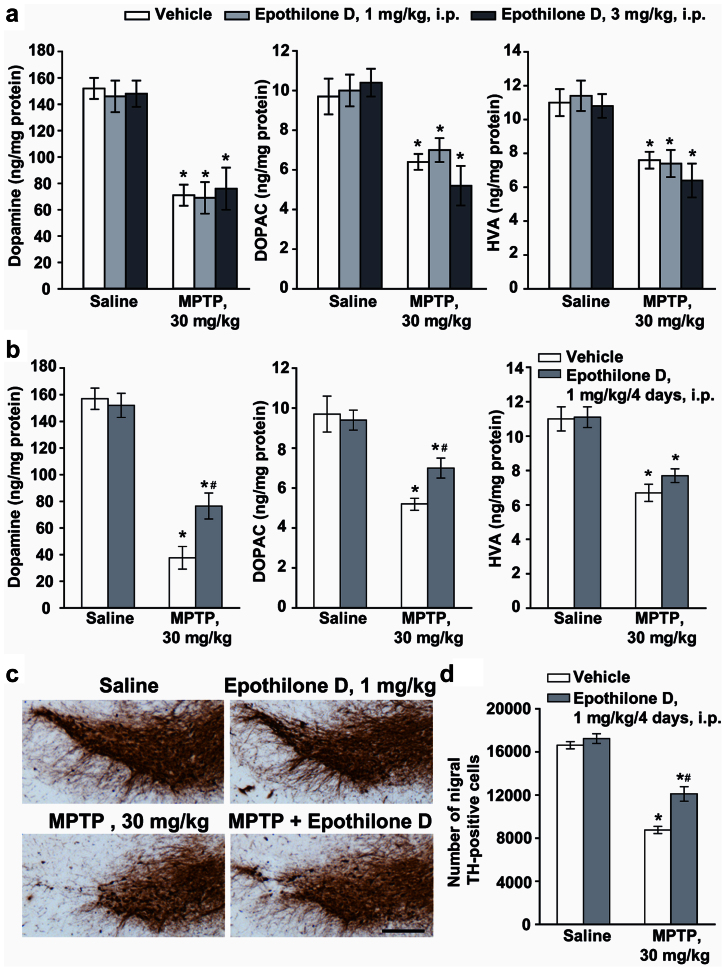
To test the hypothesis that stabilization of MT is able to exert a neuroprotective effect in experimental parkinsonism, we used the classical model induced by MPTP. C57 Black mice were challenged with a single dose of MPTP (30 mg/kg, i.p.), which led, 7 days later, to about 50% reduction in striatal DA levels. Similar reductions were found in the striatal levels of DOPAC and HVA, although changes in DOPAC and HVA levels were variable in different experiments (Fig. 6a). Reductions in DA were not affected in mice systemically injected with 1 or 3 mg/kg, i.p., of EpoD 30 min prior to MPTP (Fig. 6a). Repeated treatment with EpoD (1 mg/kg, i.p.), injected 30 min prior to MPTP and then for the following 4 days once a day, induced substantial attenuation of MPTP-induced striatal DA reduction (Fig. 6b). Note that, for unknown reasons, MPTP toxicity was greater in control animals injected with DMSO alone. Neuroprotection by repeated injections of EpoD was confirmed by stereological counts of TH-positive neurons in the pars compacta of substantia nigra (Fig. 6c,d). To assess the effects of EpoD on motor activity, mice were treated i.p. with EpoD alone (3 mg/kg, every day for four days) or 30 min prior MPTP and then every day for four days. EpoD did not affect the motor performance on the rotarod test by itself (not shown). At the doses of MPTP used, we did not observe any modification of motor performance and EpoD treatment did not modify the motor coordination at the rotarod test (not shown). We also monitored general health conditions of mice treated with EpoD and MPTP+EpoD. Both fur and body weight were not affected by EpoD or MPTP+EpoD treatments.
Figure 6. Repeated systemic injections of EpoD attenuate MPTP toxicity in mice.
(a) Biochemical analyses of striatal dopamine, DOPAC, and HVA levels in C57Bl mice injected with MPTP (30 mg/kg, i.p., single injection) alone or in combination with EpoD (1 or 3 mg/kg, i.p.) dissolved in DMSO and injected 30 min before MPTP. Mice were killed 7 days later (mean ± S.E.M., n = 8–10 mice per group). *P < 0.05; one-way ANOVA, Dunnett post hoc test versus saline-injected mice. (b) Biochemical analyses of striatal dopamine, DOPAC, and HVA levels in C57Bl mice injected with MPTP (30 mg/kg, i.p., single injection) alone or in combination with EpoD (1 mg/kg, i.p.) dissolved in DMSO and injected 30 min before MPTP and then for the following 4 days once a day. Mice were killed 7 days later (mean ± S.E.M., n = 8–10 mice per group). *, # P < 0.05; one-way ANOVA, Dunnett post hoc test versus saline-injected mice (*) or versus mice injected with MPTP alone (#). (c) Immunohistochemical analysis of TH in the pars compacta of substantia nigra of mice injected with a single i.p. injection of 30 mg/kg of MPTP, alone or combined with EpoD (1 mg/kg, i.p., 30 min prior to MPTP and then for the following 4 days, once a day). Scale bar = 250 μm. (d) Stereological counts of TH-positive cell in the substantia nigra pars compacta (mean ± S.E.M., n = 5 mice per group). *, # P < 0.05; one-way ANOVA, Dunnett post hoc test versus saline-injected mice (*) or versus mice injected with MPTP alone (#).
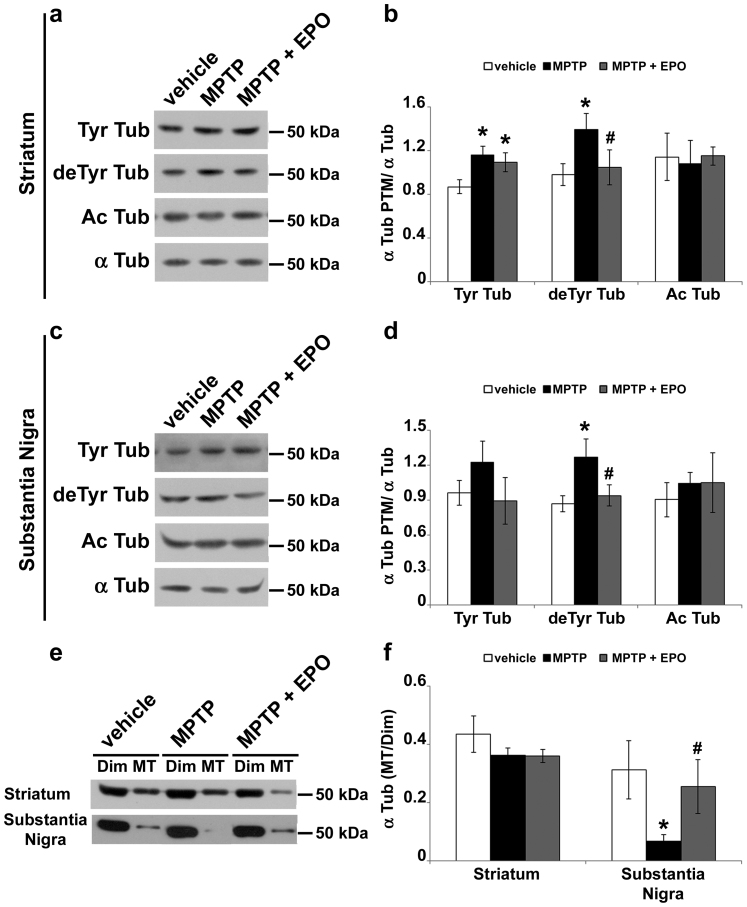
To verify that the neuprotective effect of EpoD was due to its action on MT system, we analyzed α-tubulin PTMs in the corpus striatum and substantia nigra of mice challenged with MPTP and chronically treated with EpoD. We observed significant increase of both Tyr and deTyr tubulin in the striatum of MPTP-treated mice (Fig. 7a,b), whereas only deTyr tubulin was increased by MPTP in the substantia nigra (Fig. 7c,d). Furthermore, our data show the ability of EpoD to restore deTyr tubulin baseline levels, both in striatum (Fig. 7a,b) and substantia nigra (Fig. 7c,d). The reduction in a marker of stable MTs, as deTyr tubulin is, induced by a stabilizer drug, as EpoD is, could seem surprising. However, this is perfectly consistent with the hypothesis that the increase of stable MTs in MPTP-treated mice may be an attempt to counteract the drug-induced MT destabilization18. MPP+ is known to promote MT catastrophes16 and, in respect to dynamic ones, deTyr MTs are more resistant to induced MT depolymerization; hence, tubulin detyrosination could reduce MPP+-provoked MT catastrophes. On the other side, as many other MT-stabilizing agent, EpoD directly induces MT polymerization under conditions in which tubulin is not more able to assemble, shifting the equilibrium of tubulin toward the polymeric state. To verify our hypothesis, we measured the amount of tubulin associated to cytosolic dimers and to polymeric MTs (Fig. 7e,f), both in striatum and substantia nigra. Our data reveal, for the first time, that MPTP is able to specifically destabilize MTs in the substantia nigra of treated mice and that chronic treatment with EpoD prevents the MPTP-induced destabilization (Fig. 7e,f).
Figure 7. EpoD rescues MT system in MPTP-treated mice.
(a) Immunoblot of levels of tyrosinated tubulin (Tyr Tub), detyrosinated tubulin (deTyr Tub) and acetylated tubulin (Ac Tub) in lysates of striatum of mice treated as in Fig. 6. (b) Densitometric analyses of immunoblot reported in a (mean ± S.E.M., n = 5 mice per group). For the quantitation, values of each α-tubulin PTM were normalized on the level of α-tubulin (α Tub) of the relative sample. *, # P < 0.05; one-way ANOVA, Fischer LSD post hoc test versus saline-injected mice (*) or versus mice injected with MPTP alone (#). (c) Immunoblot of levels of tyrosinated tubulin (Tyr Tub), detyrosinated tubulin (deTyr Tub) and acetylated tubulin (Ac Tub) in lysates of substantia nigra of mice treated as in Fig. 6. (d) Densitometric analyses of immunoblot reported in c (mean ± S.E.M., n = 5 mice per group). For the quantitation, values of each α-tubulin PTM were normalized on the level of α-tubulin (α Tub) of the relative sample. *, # P < 0.05; one-way ANOVA, Fischer LSD post hoc test versus saline-injected mice (*) or versus mice injected with MPTP alone (#). Tubulin dimers (Dim) and MT polymers (MT) of corpus striatum and substantia nigra were analyzed by (e) immunoblot and (f) densitometric analyses and are shown as ratio (mean ± S.E.M., n = 3 mice per group). *, # P < 0.05; one-way ANOVA, Fischer LSD post hoc test versus saline-injected mice (*) or versus mice injected with MPTP alone (#).
Discussion
Cytoskeletal alterations have been described in many central nervous system disorders, but it is unclear whether they are a cause or simply a by-product of neurodegeneration. Here, we show that systemic injection of MPTP in mice induces MT dysfunction that occurs very early, before axonal transport impairment, TH depletion, and, ultimately, dopaminergic neuron degeneration. Noteworthy is that chronic administration of the MT stabilizer Epo D rescues MT defects and is neuroprotective, supplying reliable proof that MT dysfunction may contribute to actually cause neurodegeneration.
Axonal transport impairment in the MPTP model of PD was first suggested by Morfini et al. (2007), who proposed that it was an early event in neurodegeneration, based on the outcome of a study on giant squid axons. Here we demonstrate that axonal transport impairment occurs in MPTP-treated mice, showing changes in mitochondria distribution in dopaminergic fibres. In addition, the earlier decrease in TH in striatum, followed by TH depletion in substantia nigra, can easily be explained by impairment of axonal transport, other than by the dying back mechanism of degeneration, typical of PD12 and mice exposed to MPTP34. Looking for the mechanisms underlying axonal transport impairment, we found that MPTP does not impact motor protein levels. However, it affects the levels of deTyr MTs, suggesting that altered MT stability is responsible for the alterations of axonal transport. Indeed, Kinesin 1, which is particularly abundant in neurons, is preferentially recruited, but moves slowly along highly modified MTs33. Furthermore, MPP+ induces increases in deTyr tubulin content that precede and therefore may cause the reduction in mitochondrial transport in PC12 cells18. Consequently, we supposed that a similar scenario is likely to occur in MPTP-treated mice. Nevertheless, another paper by O'Malley's group26 reported that MPP+ specifically impairs mitochondria transport in dopaminergic neurons, an event that precedes autophagy and MT defects. However, they looked at gross, i.e. Ac-MT fragmentation and α-tubulin content reduction, rather than at subtle alterations of MTs, such as changes in PTMs. A further possible cause of the axonal transport block is MT reorientation, which is induced either by MPP+ 18 or by human mutant Tau expression35 leading to traffic jams. Therefore, the observed impairment of axonal transport is likely mediated by alterations of MT stability and organization, which, in turn, lead to distal axon degeneration, as dying back is, and to the accumulation of DA loaded vesicles in cell soma. This could really be detrimental to the neurons since DA oxidation produces large quantities of reactive oxygen species (ROS) triggering dopaminergic neuron death36.
The observation that levels of the neuron-specific βIII tubulin, which is the most dynamic among the β-tubulin isotypes37, are increased points out the importance of MT dynamics and its tight regulation in MPTP-mediated neurodegeneration, as we have already shown16,18. βIII tubulin enrichment could be explained as an adaptive mechanism to counteract the MPTP-induced reduction in Tyr α−tubulin, usually associated with highly dynamic MTs. Moreover, βIII tubulin expression is primarily restricted to the nervous system38, and it has been suggested that different β-tubulin isotypes could serve specific and unique roles39, such as neuronal elongation and axon guidance. Banarjee and colleagues40 showed that in the presence of Tau, a neuron-specific MT binding protein, βIII tubulin was more prone to polymerize than the other isotypes. Worthy of note is the recent suggestion that the Tau-MTs interaction may be important not only in the pathogenesis of Alzheimer's disease, but also of PD. Indeed, both MPTP and α-synuclein mutations promote Tau phosphorylation, causing MT instability, which leads to loss of dopaminergic neurons in PD brain41. Nevertheless, trying to counteract the ongoing axonal destruction, the dopaminergic neurons could promote MT polymerization through increase in the βIII tubulin content or in MT stability, as suggested by the enrichment in deTyr and Ac α-tubulin.
Mitochondria are largely considered crucial players in the pathogenesis of PD. Indeed, both MPP+ 42 and rotenone43 inhibit mitochondrial complex I, reducing ATP synthesis and increasing ROS production. However, the lack of complex I does not protect dopaminergic neurons from toxin administration44, suggesting the existence of alternative mechanisms of action. It is well known that tubulin interacts with VDAC, the most abundant protein in the mitochondrial outer membrane, and mitochondria-associated tubulin is enriched in βIII isotype45. Recently, it has been reported that tubulin decreases the respiration rate of isolated mitochondria46 and that the increase in tubulin dimers induces mitochondrial depolarization in human cancer cells47. Furthermore, the administration of tubulin-targeted drugs induces mitochondrial depolarization and Ca++ release48. This body of evidence clearly shows that interfering with MT system impairs mitochondria activity. Therefore, enrichment in free tubulin dimers in PC12 cells18 and MPTP-induced increase in βIII tubulin (present data) could induce adverse effects, such as mitochondrial dysfunction. Being life a matter of balance, when the equilibrium shifts from a beneficial event, such as MT polymerization induced by βIII tubulin increases, toward a detrimental one, such as mitochondrial dysfunction caused by the same factor, dopaminergic neurons may die leading to PD. In this context, it is essential to consider parkin, an E3 ligase promoting degradation of tubulin and other proteins, known to interact with MTs and to play a central role in the regulation of mitophagy. Thanks to its particular position, parkin may quarantine damaged mitochondria, by severing their connection to the MT network, before promoting their clearance49. If MT-parkin interaction was impaired, as in the case of MT destabilization, proper regulation of mitophagy would fail, leading to dopaminergic neuron loss. Thus, tubulin partitioning between dimer and polymer pools regulates multiple steps in mitochondrial metabolism and, therefore, in the control of neuronal health and death.
A first step designed to block the progression of the disease would be the regeneration of collapsing axons. MT stabilization could be useful to physically counteract axon disruption, reinforcing the pillars that support the structures, and to prevent mitochondrial damage, reducing the level of free tubulin dimers. In fact, the activation of signal cascades that converge on MT stability modulation has trophic effects on axon formation in vivo50,51. It has already been shown that the MT-stabilizer Taxol protects cultured dopaminergic neurons against rotenone toxicity17,52, reduces scarring formation and promotes regeneration of central nervous system axons53,54. Unfortunately, the blood-brain barrier penetration of Taxol is very poor55. EpoD, another MT-stabilizing compound, penetrates through the blood-brain barrier and has resulted to be neuroprotective in mouse models of schizophrenia56 and tauopathy19. Moreover, it improves axonal MT density, reduces axonal dystrophy and alleviates cognitive deficits in transgenic mouse models of tauopathies19,20. What is more, the dynamicity of MTs is increased in tau transgenic mice and treatment with EpoD restored MT dynamics. MT stabilization had beneficial effects on behavior, tau pathology and neurodegeneration21. Here, we used the classical model of experimental parkinsonism induced by MPTP to test the hypothesis that MT stabilization is also able to counteract degeneration in another model of neurodegeneration. Acute injections of EpoD did not affect the toxic effect induced by MPTP, whereas repeated injections of EpoD restored tubulin PTMs and MT mass in the substantia nigra and exerted neuroprotective effects in the dopaminergic nigrostriatal system of MPTP-treated mice. Therefore, doses of EpoD, which were chosen according to Barten and colleagues21, are also effective in the MPTP model of dopaminergic neurotoxicity. These data pinpoint that alterations of ΜΤs are very early events, which specifically occur in dopaminergic neurons in experimental parkinsonism, and reinforce the idea that the cytoskeleton of dopaminergic neurons is particularly vulnerable but is also highly responsive to MT-targeting agents.
Taken together with our recent findings that MT stability is impaired in human fibroblasts deriving from PD patients and that MT stabilization rescues control phenotype28, the present work suggests that MTs are a potential target for pharmacological therapy designed to block the axonal disruption leading to PD. Thus, chronic pharmacological stabilization of MT may be a viable strategy for the management of the disease.
Methods
Materials
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) was purchased from Sigma (St. Louis, MO). Epothilone D was purchased from Acme Bioscience (Palo Alto, CA).
Animals
Male C57 Black mice (22–24 g, b.w., 8–9 week old) were purchased from Charles River (Calco, Italy) and used for all experiments. Mice were kept under environmentally controlled conditions (ambient temperature = 22°C, humidity = 40%) on a 12-h light/dark cycle with food and water ad libitum. The study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Italian Institute of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the I.R.C.C.S. Neuromed Institute. Permit Number 432007/A was issued by the Italian Ministry of Health. All efforts were made to minimize suffering.
Treatments
Mice were treated with one single i.p. injection of 36 mg/kg of MPTP (corresponding to 30 mg/kg of free MPTP) or with three doses of 24 mg/kg of MPTP hydrochloride (corresponding to 20 mg/kg of free MPTP), injected i.p. at 2 h intervals (cumulative dose = 60 mg/kg of free MPTP). Twelve or 72 h after last injection of MPTP, mice were killed by decapitation or by intracardiac perfusion, to perform biochemical or immunohistochemical analysis, respectively. One cerebral hemisphere of mice used for biochemical analysis, was used to evaluate levels of striatal DA and its metabolites, 3,5-dihydroxyphenylactic acid (DOPAC) and homovanillic acid (HVA). For EpoD experiments, mice were injected with a single i.p. dose of 36 mg/kg of MPTP and then acutely (1 or 3 mg/kg, i.p., 30 min prior to MPTP) or chronically treated (1 mg/kg, i.p., 30 min prior to MPTP and then every day for 4 days) with EpoD. Mice were killed 7 days later for biochemical analysis of MTs and biochemical and immunohistochemical assessment of nigro-striatal damage. EpoD was dissolved in DMSO and injected i.p. (in a volume of 50 μl/mouse). Control mice were injected with the vehicle alone.
Motor activity assessment
Motor coordination was assessed by the rotarod test. The rotarod apparatus consisted of a motor driver control unit (Ugo Basile, Varese, Italy) and a rotating horizontal cylinder (30 mm), divided into five separate rotating compartments and fully enclosed to ensure that the mice did not jump out of their area. Automatic timers recorded the time (in seconds) the mice remained on the rod, which was rotating at an accelerating speed from 5 to 15 rpm. Mice were tested for 10 min on the rotarod every day, starting 2 days after MPTP or EpoD injection (as above). General health conditions (fur, body weight and mortality) were also monitored by an experimenter unaware of treatments.
Monoamine assay
The corpus striatum was immediately dissected out homogenized by sonication in 0.6 ml of ice-cold 0.1 M PCA. Fifty μl of the homogenate were used for protein determination57. The remaining aliquot was centrifuged at 8,000 g for 10 min, and 20 μl of the supernatant was injected into an HPLC equipped with autosampler 507 (Beckman Instruments, Fullerton, CA), a programmable solvent module 126 (Beckman), an analytical C-18 reverse-phase column kept at 30°C (Ultrasphere ODS 5 μm, 80 Å pore, 250 × 4.6 mm (Beckman), and a Coulochem II electrochemical detector (ESA, Inc., Chelmsford, MA). The holding potentials were set at +350 and −350 mV for the detection of DA, DOPAC and HVA. The mobile phase consisted of 80 mM sodium phosphate, 40 mM citric acid, 0.4 mM EDTA, 3 mM 1-heptansulphonic acid and 8.5% methanol, brought to pH 2.75 with phosphoric acid (run under isocratic conditions, at 1 ml/min).
Western blot analysis
Western blot analysis was performed on protein extracts or cytoskeletal fractions obtained from mouse brain regions. To get total proteins, corpus striatum and substantia nigra were immediately dissected out on ice, mechanically homogenized and, subsequently, sonicated in SDS-PAGE sample buffer. Separation of cytosolic tubulin dimers from MT polymers was performed accordingly to Fanara and colleagues58. Briefly, corpus striatum and substantia nigra were gently homogenized in MT-stabilizing buffer and postnuclear supernatants were centrifuged at 200,000 g at 20°C for 20 min; the supernatant (containing the soluble dimeric tubulin) and the pellet (containing the MT fraction) were separated and stored at −20°C. Western blots were made as previously described18 using the following antibodies: α-tubulin mouse IgG (clone B-5-1-2, Sigma-Aldrich); deTyr tubulin rabbit IgG (Chemicon, Temecula, CA); Tyr tubulin mouse IgG (clone TUB-1A2, Sigma-Aldrich); Ac tubulin mouse IgG (clone 6-11B-1, Sigma-Aldrich); β-tubulin mouse IgG (clone Tub 2.1, Sigma-Aldrich); βΙΙΙ tubulin mouse IgG (clone TU-20, kindly provided by Dr. Pavel Dràber, Prague, Czec Republic); actin mouse IgM (N350, Amersham, Little Chalfont, UK); tyrosine hydroxylase (TH) mouse IgG (clone 6dt, Abcam, Cambride, UK) 1:600; kinesin rabbit IgG (Abcam) 1:1000; Dynein mouse IgG (clone 74.1, Millipore) 1:500, GAPDH (Abcam). Membranes were washed for 30 min with 3 changes and incubated for 1 h at room temperature with HRP donkey anti-mouse IgG (Pierce), HRP goat anti-mouse IgM (Sigma-Aldrich), or HRP goat anti-rabbit IgG (Pierce). Immunostaining was revealed by enhanced chemiluminescence (Super-Signal West Pico Chemiluminescent, Pierce). Quantification was performed by ImageJ software (NIH, Bethesda, MD).
Confocal analysis
Mice were anesthetized with chloralium hydrate (320 mg/kg, i.p.) and transcardially perfused with 4% PFA in 0.1 M phosphate buffer, pH 7.4. Brains were removed, postfixed overnight in 4% PFA, and then transferred in 30% sucrose for cryoprotection. Sagittal sections (50 μm thick) were cut with a Vibratome (VT1000S, Leica). Sections were stained with porin rabbit IgG (VDAC1/porin, Abcam, Cambride, UK) and the following antibodies previously used for immmunoblotting: deTyr tubulin rabbit IgG; Tyr tubulin mouse IgG; Ac tubulin mouse IgG; porin rabbit IgG (VDAC1/porin, Abcam). To identify dopaminergic neurons and fibres, each section was concurrently stained with anti-TH antibody, made in mice (clone LCN1, Millipore) or rabbits (Millipore) as appropriate. As secondary antibodies we used Alexa Fluor™ 568 donkey anti-mouse IgG, and Alexa Fluor™ 488 goat anti-rabbit IgG (Invitrogen). Coverslips were mounted in PBS-glycerol and examined with a confocal laser scan microscope imaging system (TCS SP2 AOBS, Leica Microsystems, Heidelberg, Germany) equipped with an Ar/Ar-Kr 488 nm, 561 nm and 405 nm diode lasers. Photomultiplier gain for each channel was adjusted to minimize background noise and saturated pixels and, once defined for control conditions, parameters were kept constant for all acquisitions. To estimate the overlapping area between red and green signals, analyses were carried out on single-plane raw images and Manders' coefficients were calculated applying the JACoP plug-in (developed and reviewed by 31) for ImageJ software. To evaluate the mitochondria distribution, the porin signal was superimposed on dopaminergic fibres, using the Mask tool of the Leica Confocal Software (Leica); mitochondria accumulations were identified as white pixels-containing area, as thick as long, clearly separated from other white pixels. TH-positive signal longer than 5 μm was considered as dopaminergic fibre, and signals separated by more than 10 μm were counted as two distinct fibres.
Immunohistochemical analysis of tyrosine hydroxylase
Brains were removed, fixed in ethanol (60%), acetic acid (10%), and chloroform (30%), and included in paraffin. Tissue sections (30 μm) were incubated overnight with monoclonal mouse anti-TH (1:200; Sigma-Aldrich) and then for 1 h with secondary biotin-coupled anti-mouse antibodies (1:200; Vector Laboratories, Burlingame, CA). 3,3-Diaminobenzidine tetrachloride (Sigma) was used for detection.
Stereological cell counting of tyrosine hydroxylase-positive cells in the substantia nigra pars compacta
The number of TH-positive cells in the substantia nigra pars compacta was assessed by stereological technique and an optical fractionator using a Zeiss Axio Imager M1 microscope equipped with a motorized stage and focus control system (Zeta axis), and with a digital video camera. The software Image-Pro Plus 6.2 for Windows (Media Cybernetics, Inc., Bethesda, MD) equipped with a Macro was used for the analysis of digital images. The Macro was obtained by “Immagini e Computer” (Bareggio, Italy). The characteristics of this Macro are published59. The analysis was performed on 6 sections of 20 μm, sampled every 200 μm on the rostro-caudal extension, in which the substantia nigra was identified and outlined at 2.5× magnification. TH-positive cells were counted at 100× magnification as described60. For stereological analysis, we used a grid of disectors (counting frame of 100 × 100 μm; grid size 50 × 50 μm), with 1.3 as numerical aperture of the lens. The total number of TH-positive cells in the substantia nigra was computed from the formula: N = Σ(n) × 1/SSF × 1/ASF × 1/TSF, where n is the total number of cells counted on each disector; SSF (fraction of sections sampled) the number of regularly spaced sections used for counts divided by the total number of sections across the substantia nigra pars compacta; ASF (area sampling frequency) the disector area divided by the area between disectors (7500 μm2 × disector number/region area); and TSF (thickness sampling frequency) the disector thickness divided by the section thickness (20 μm).
Statistical analysis and data managing
The statistical significance of treatment was assessed by one-way ANOVA with Dunnett 2-sided or Fischer LSD post-hoc testing or χ2 test when appropriate. Analyses were performed using STATISTICA (StatSoft Inc., Tulsa, OK).
Author Contributions
D.C. performed protein analysis by Western blotting and confocal microscopy analysis; F.C. performed immunohistochemistry; C.L.B. and G.M. performed in vivo experiments and prepared tissue for protein analysis; D.B. performed immunohistochemistry and stereological counts of tyrosine hydroxylase-positive cells; A.T. performed biochemical analysis of striatal dopamine and its metabolite levels; D.P. and E.G. designed experiments; D.C., G.P., G.B. and C.G. designed the experiments, analysed the data and wrote the paper.
Supplementary Material
Supplementary figures and tables
Acknowledgments
The authors are grateful to Dr. Alida Amadeo and Dr. Carmelita De Gregorio (Università degli Studi di Milano) for technical support and helpful discussions, and Dr. Jennifer S. Hartwig for reading and editing the manuscript. This work was supported by Fondazione Grigioni per il Morbo di Parkinson, Milan, Italy (to G.C.), and “Dote ricerca”, FSE, Regione Lombardia (to D.C.).
References
- Sato M., Schwart W. H., Selden S. C. & Pollard T. D. Mechanical properties of brain tubulin and microtubules. J. Cell. Biol. 106, 1205–1211 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T. & Kirschener M. Dynamic instability of microtubule growth. Nature 312, 237–242 (1984). [DOI] [PubMed] [Google Scholar]
- Inoué S. & Salmon E. D. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol. Biol. Cell 6, 1619–1640 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde C. & Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 10, 319–332 (2009). [DOI] [PubMed] [Google Scholar]
- Baas P. W. & Black M. M. Individual microtubules in the axon consist of domain that differ both in composition and stability. J. Cell Biol. 111, 495–509 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta N., Orso G., Rosetto M. G., Draga A. & Broasle K. The ereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Neuron 14, 1135–1147 (2004). [DOI] [PubMed] [Google Scholar]
- Janke C. & Kneussel M. Tubulin post-translational modifications: encoding functions on the neuronal microtubule cytoskeleton. Trends Neurosci. 33, 362–372 (2010). [DOI] [PubMed] [Google Scholar]
- Raff M. C., Whitmore A. V. & Finn J. T. Axonal self-destruction and neurodegeneration. Science 296, 868–871 (2002). [DOI] [PubMed] [Google Scholar]
- Zhai Q. et al. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron 39, 217–225 (2003). [DOI] [PubMed] [Google Scholar]
- Ertürk A., Hellal F., Enes J. & Bradke F. Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. J. Neurosci. 27, 9169–9180 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Yu W. & Baas P. W. Microtubule reconfiguration during axonal retraction induced by nitric oxide. J. Neurosci. 22, 5982–5991 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W. & Przedborski S. Parkinson's disease: mechanisms and models. Neuron 39, 889–909 (2003). [DOI] [PubMed] [Google Scholar]
- Alim M. A. et al. Demonstration of a role for alpha-synuclein as a functional microtubule-associated protein. J. Alzheimer's Dis. 6, 435–442 (2004). [DOI] [PubMed] [Google Scholar]
- Yang F. et al. Parkin stabilizes microtubules through strong binding mediated by three independent domains. J. Biol. Chem. 280, 17154–17162 (2005). [DOI] [PubMed] [Google Scholar]
- Gillardon F. Leucine-rich repeat kinase 2 phosphorylates brain tubulin-β isoform and modulates microtubule stability: a point of convergence in parkinsonian neurodegeneration? J. Neurochem. 110, 1514–1522 (2009). [DOI] [PubMed] [Google Scholar]
- Cappelletti G., Surrey T. & Maci R. The parkinsonism producing neurotoxin MPP+ affects microtubule dynamics by acting as destabilising factor. FEBS Lett. 579, 4781–4786 (2005). [DOI] [PubMed] [Google Scholar]
- Ren Y., Liu W., Jiang H., Jiang Q. & Feng J. Selective vulnerability of dopaminergic neurons to microtubule depolymerization. J. Biol. Chem. 280, 34105–34112 (2005). [DOI] [PubMed] [Google Scholar]
- Cartelli D. et al. Microtubule dysfunction precedes transport impairment and mitochondria damage in MPP+-induced neurodegeneration. J. Neurochem. 115, 247–258 (2010). [DOI] [PubMed] [Google Scholar]
- Brunden K. R. et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J. Neurosci. 30, 13861–13866 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B. et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J. Neurosci. 32, 3601–3611 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barten D. M. et al. Hyperdynamic microtubules, cognitive deficits, and pathology are improved in tau transgenic mice with low doses of the microtubule-stabilizing agent BMS-241027. J. Neurosci. 32, 7137–7145 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G. et al. Pharmacological activation of mGlu4 metabotropic glutamate receptors reduces nigrostriatal degeneration in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J. Neurosci. 26, 7222–7229 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ara J. et al. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Proc. Natl. Acad. Sci. USA 95, 7659–7663 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos K. J., Grierson A. J., Ackerley S. & Miller C. C. Role of axonal transport in neurodegenerative diseases. Annu. Rev. Neurosci. 31, 151–173 (2008). [DOI] [PubMed] [Google Scholar]
- Morfini G. et al. 1-methyl-4-phenylpyridinium affects fast axonal transport by activation of caspase and protein kinase C. Proc. Natl. Acad. Sci. USA 104, 2442–2447 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kin-Han J. S., Antenor-Dorsey J. A. & O'Malley K. L. The parkinsonian mimetic, MPP+, specifically impairs mitochondrial transport in dopaminergic axons. J. Neurosci. 31, 7212–7221 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. Y., Koprich J. B., Siddigi H. & Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precedes dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J. Neurosci. 29, 3365–3373 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartelli D., Goldwurm S., Casagrande F., Pezzoli G. & Cappelletti G. Microtubule destabilization is shared by genetic and idiopathic Parkinson's disease patients fibroblasts. PLoS One 7, e37467 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Niwa S. & Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron 68, 610–638 (2010). [DOI] [PubMed] [Google Scholar]
- Rogowski K. et al. A Family of Protein-Deglutamylating Enzymes Associated with Neurodegeneration. Cell 143, 564–578 (2010). [DOI] [PubMed] [Google Scholar]
- Bolte S. & Cordelières F. P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 (2006). [DOI] [PubMed] [Google Scholar]
- Reed N. A. et al. Microtubule acetylation promotes kinesin-1 binding and transport. Curr. Biol. 16, 2166–2172 (2006). [DOI] [PubMed] [Google Scholar]
- Dunn S. et al. Differential trafficking of Kif5c on tyrosinated and detyrosinated in live cells. J. Cell. Sci. 121, 1085–1095 (2008). [DOI] [PubMed] [Google Scholar]
- Cochiolo J. A., Ehsanian R. & Bruck D. K. Acute ultrastructural effect of MPTP on the nigrostriatal pathway of the C57Bl/6 adult mouse: evidence of compensatory plasticity in nigrostriatal neurons. J. Neurosci. Res. 59, 126–135 (2000). [PubMed] [Google Scholar]
- Shemesh O. A., Erez H., Ginzburg I. & Spira M. E. Tau-induced traffic jams reflect organelles accumulation at points of microtubule polar mismatching. Traffic 9, 458–471 (2008). [DOI] [PubMed] [Google Scholar]
- Hastings T. G., Lewis D. A. & Zigmond M. J. Role of the oxidation in the neurotoxic effect of intrastriatal dopamine injections. Proc. Natl. Acad. Sci. USA 93, 1956–1961 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda D., Miller H. P., Banerjee A., Ludueña R. F. & Wilson L. Microtubule dynamics in vitro are regulated by the tubulin isotype composition. Proc. Natl. Acad. Sci. USA 91, 11358–11362 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsetos C. D., Herman M. M. & Mörk S. J. Class III beta-tubulin in human development and cancer. Cell. Motil. Cytoskeleton 55, 77–96 (2003). [DOI] [PubMed] [Google Scholar]
- Ludueña R. F. Are tubulin isotypes functionally significant. Mol. Biol. Cell 4, 445–57 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., Roach M. C., Trcka P. & Luduena R. F. Preparation of a monoclonal antibody specific for the class IV isotype of beta-tubulin. Purification and assembly of alpha beta II, alpha beta III, and alpha beta IV tubulin dimers from bovine brain. J. Biol. Chem. 267, 5625–5630 (1992). [PubMed] [Google Scholar]
- Qureshi H. Y. & Paudel H. K. Parkinsonian neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and alpha-synuclein mutations promote Tau protein phosphorylation at Ser262 and destabilize microtubule cytoskeleton in vitro. J. Biol. Chem. 286, 5055–5068 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas W. J., Vyas I. & Hekkila R. E. Inhibition of NADH-linked oxidation in brain mitochondria by MPP+, a metabolite of the neurotoxin MPTP. Life Sci. 36, 2503–2508 (1985). [DOI] [PubMed] [Google Scholar]
- Betabert R. et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 3, 1301–1306 (2000). [DOI] [PubMed] [Google Scholar]
- Choi W. S., Kruse S. E., Palmiter R. D. & Xia Z. Mitochondrial complex I inhibition is not required for dominergic neurons death induced by rotenone, MPP+, or paraquat. Proc. Natl. Acad. Sci. USA 105, 15136–15141 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré M. et al. Tubulin is an inherent component of mitochondrial membranes that interacts with the voltage-dependent anion channel. J. Biol. Chem. 277, 33664–33669 (2002). [DOI] [PubMed] [Google Scholar]
- Rostovtseva T. K. et al. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc. Natl. Acad. Sci. USA 105, 18746–18751 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado E. N., Patnaik J., Mullins M. R. & Lemasters J. J. Free tubulin modulates mitochondrial membrane potential in cancer cells. Cancer Res. 70, 10192–10201 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov S. L., Ivannikov M. V. & Johansson M. [Ca2+]i signaling between mitochondria and endoplasmic reticulum in neurons is regulated by microtubules. From mitochondrial permeability transition pore to Ca2+-induced Ca2+ release. J. Biol. Chem. 280, 715–721 (2005). [DOI] [PubMed] [Google Scholar]
- Wang X. PINK1 and Parkin Target Miro for Phosphorylation and Degradation to Arrest Mitochondrial Motility. Cell 147, 893–906 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries V. et al. Oncoprotein Akt/PKB induces trophic effects in murine models of Parkinson's disease. Proc. Natl. Acad. Sci. USA 103, 18757–18762 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S., Banba Y., Satake T. & Ohno S. Axon formation in neocortical neurons depends on stage-specific regulation of microtubule stability by the dual leucine zipper kinase-c-Jun N-terminal kinase pathway. J. Neurosci. 31, 6468–6480 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W. S., Palmiter R. D. & Xia Z. Loss of mitochondrial complex I activity potentiates dopamine neuron death induced by microtubule dysfunction in a Parkinson's disease model. J. Cell. Biol. 192, 873–882 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellal F. et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science 331, 928–931 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengottuvel V., Leibinger M., Pfreimer M., Andreadaki A. & Fischer D. Taxol facilitates axon regeneration in the mature CNS. J. Neurosci. 31, 2688–2699 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner S. et al. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J. Clin. Invest. 110, 1309–1318 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrieux A. et al. Microtubule stabilizer ameliorates synaptic function and behavior in a mouse model for schizophrenia. Biol. Psychiatry 60, 1224–1230 (2006). [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Rosebrough N. Y., Farr A. L. & Randall R. Y. Protein measurement with Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951). [PubMed] [Google Scholar]
- Fanara P. et al. In vivo measurment of microtubule dynamics using stable isotyoe labeling with heavy water. J. Biol. Chem. 279, 49940–49947 (2004). [DOI] [PubMed] [Google Scholar]
- King M. A., Scotty N., Klein R. L. & Meyer E. M. Particle detection, number estimation, and feature measurement in gene transfer studies: optical fractionator stereology integrated with digital image processing and analysis. Methods 28, 293–299 (2002). [DOI] [PubMed] [Google Scholar]
- Gundersen H. J. G. & Jensen E. B. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 147, 229–263 (1987). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and tables