Table 2.
Reactions of structurally varied amines wtih 6.a
| entry | amine | product | time | yieldb |
|---|---|---|---|---|
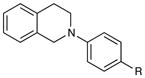
|
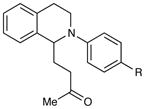
|
|||
| 1 | R = H | 5 h | 90% | |
| 2 | R = Me | 3 h | 97% | |
| 3 | R = OMe | 24 h | 94% | |
| 4 | R = Cl | 18 h | 99% | |
| 5 |
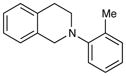
|
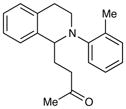
|
72 h | 12%c |
| 6 |
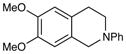
|
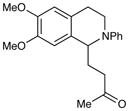
|
24 h | 97% |
| 7 |
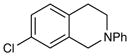
|
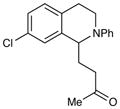
|
18 h | 96% |
| 8 |
|
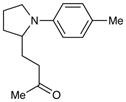
|
36 h | 30%c |
Unless otherwise noted, reactions were conducted using 2 mol% Ru(bpy)3Cl2, 2 equiv of MVK, and 1 equiv of TFA in degassed MeCN (0.25 M) at 50 °C and were irradiated using a 23 W compact fluorescent light bulb at a distance of 30 cm.
Values represent the averaged isolated yields of two reproducible experiments unless otherwise noted.
Yield determined by 1H NMR using an internal standard.
