Table 3.
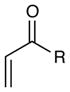
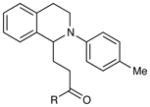
Reactions of Michael acceptors with tetrahydroisoquinoline 5.a
| entry | enone | product | time | yieldb (d.r.)c |
|---|---|---|---|---|
|
|
|||
| 1 | R = Et | 4 h | 99% | |
| 2 | R = Ph | 2 h | 91% | |
| 3 | R = OMe | 1 h | 12%d | |
| 4 | R = H | 1 h | 96% | |
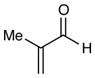
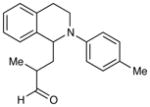
| 5 |
|
|
5 h | 93% (2:1 d.r.) |
| 6 |
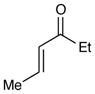
|
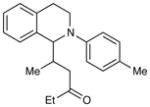
|
72 h | 46%d (2:1 d.r.) |
Unless otherwise noted, reactions were conducted using 2 mol% Ru(bpy)3Cl2, 2 equiv of MVK, and 1 equiv of TFA in degassed MeCN (0.25 M) at 50 °C and were irradiated using a 23 W compact fluorescent light bulb at a distance of 30 cm.
Values represent the averaged isolated yields of two reproducible experiments unless otherwise noted.
Diastereomer ratios determined by 1H NMR.
Yield determined by 1H NMR using an internal standard.
