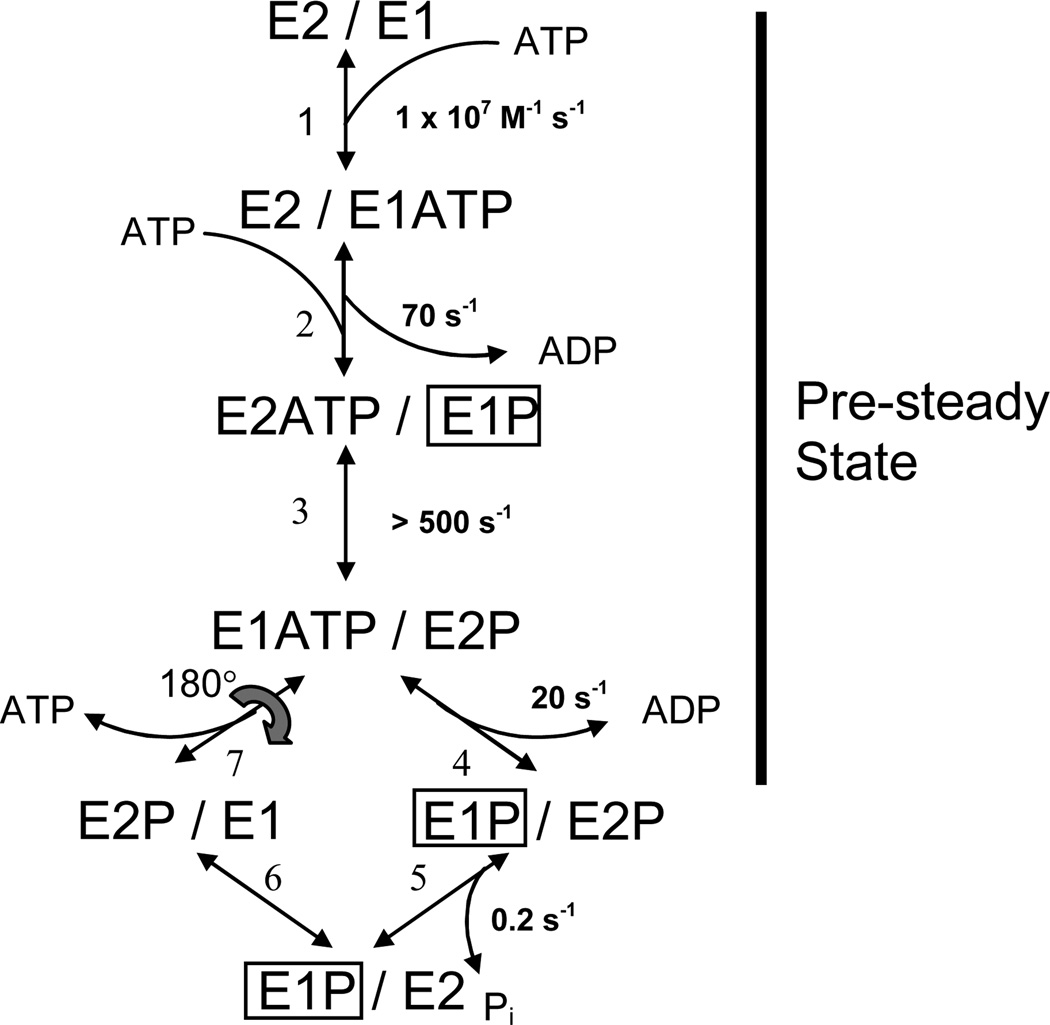
Fig. 1.
Dimer model of the skeletal SR Ca2+-ATPase (SERCA1). The model consists of parallel catalytic pathways constrained to out-of-phase (asynchronous) cycling by conformational coupling between the subunits. The subunits oligomerize forming an asymmetric E2/E1 dimer in which the right-hand (leading) subunit completes the reactions of the catalytic cycle ahead of the left-hand (lagging) subunit. Completion of steps 1–4 produces an asymmetric phosphorylated 2dimer, E1P/E2P, which accumulates because of slow dephosphorylation in the subsequent step. The steady state portion of the cycle (steps 5–7) regenerates the product of step 3, completing the loop. Rate constants assigned to the reactions represent values obtained from quenched-flow experiments conducted at 2°C and 0.1 M KCl (3). ADP-sensitive phosphoenzyme (E1P) inside the box is associated with the transient EPR signal that arises from the weakly-immobilized spin label fraction in [2H]IASL-Ca2+-ATPase. Rapid turnover (>500 s−1) of E1P in the leading subunit explains the failure to detect its corresponding EPR signal.