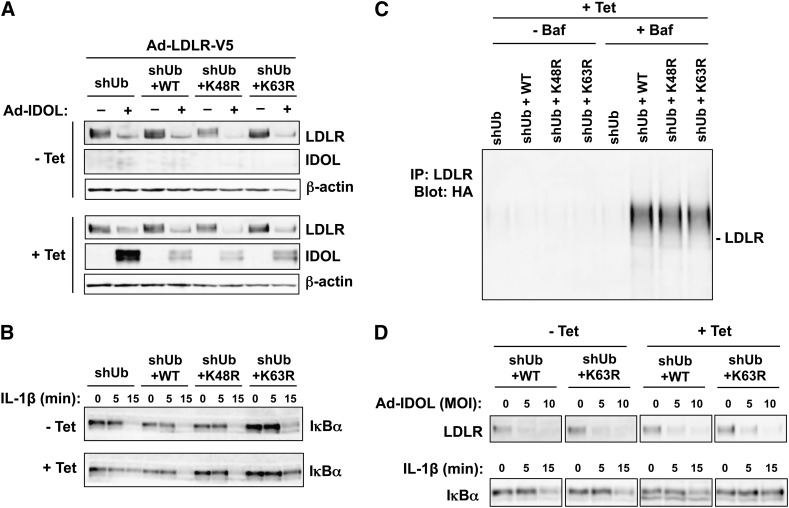
Fig. 5.
The ubiquitination and degradation of the LDLR do not require K48- or K63-specific ubiquitin linkages. A: U2OS cell lines were cultured in the absence (−) or presence (+) of 1 μg/ml tetracycline (Tet) for 48 h before being infected with adenoviral vectors encoding LDLR and/or IDOL as indicated. Cells were lysed in RIPA buffer for 24 h after adenovirus infection. Proteins in the lysates were immunoblotted with the indicated antibodies. B: U2OS cell lines were cultured in the absence or presence of 1 μg/ml tetracycline for 72 h and treated with 10 ng/ml IL-1β for the indicated times before being lysed in RIPA buffer. Proteins in the lysates were analyzed by immunoblotting. C: U2OS cells were cultured in the presence of 1 μg/ml tetracycline for 48 h before infections with adenoviral vectors encoding IDOL and V5-tagged LDLR. Cells were lysed in RIPA buffer for 24 h after the adenovirus infection, with the last 4 h in the presence of 50 nM bafilomycin (Baf). Proteins in the lysates were immunoprecipitated with an antibody recognizing LDLR and immunoblotted with HA antibody. D: Upper panel: U2OS cell lines were cultured in the absence or presence of 1 μg/ml tetracycline for 96 h before coinfection with adenovirus encoding the LDLR and the indicated titers of adenovirus encoding IDOL. Cells were lysed in RIPA buffer for 24 h after adenovirus infection. Proteins in the lysates were immunoblotted with the indicated antibody. Lower panel: U2OS cells were cultured in the absence or presence of 1 μg/ml tetracycline for 120 h and treated with 10 ng/ml IL-1β for the indicated times before lysis in RIPA buffer. Proteins in the lysates were immunoblotted with the indicated antibody.