Abstract
Transcatheter aortic valve implantation (TAVI) is becoming a valuable alternative to surgical aortic valve replacement in non-operable and high-risk surgical patients. As the population of heart donors and recipients ages, the prevalence of degenerative valvular disease after transplantation will increase. The optimal treatment strategy of valvulopathies in these patients with extensive comorbidity is still unknown because of insufficient published experience. We present a heart transplant recipient with renal failure, systolic heart failure and severe aortic stenosis who was successfully treated with transapical TAVI.
Keywords: Transcatheter aortic valve implantation, Transplant, Heart failure
INTRODUCTION
Aortic valve stenosis is the most frequent valvular disease in the western aging population. Untreated symptomatic aortic valve stenosis carries a bad prognosis, and surgical aortic valve replacement (AVR) has been the mainstay therapy for decades. Recently, transcatheter aortic valve implantation (TAVI) has shown to offer a valuable treatment alternative in high-risk patients and those who are deemed to be inoperable [1, 2].
Heart transplantation is a treatment of advanced heart failure with median survival of >10 years in international registries [3]. As the population of heart transplant recipients as well as heart donor ages, the prevalence of degenerative valvular disease in combination with severe comorbidity after heart transplantation is expected to increase. This will confront us with difficult choices concerning treatment strategies. We present a heart transplant recipient with renal failure, moderate abdominal aortic aneurysm, femoral arterial disease, systolic heart failure and severe aortic stenosis who was successfully treated with transapical TAVI.
CASE
In 1993, a 59-year old patient received a heart transplantation for an ischaemic cardiomyopathy. Because of biventricular primary non-function of the graft, he was treated with a biventricular assist device (Abiomed) and retransplantation. Later, the patient developed severe transplant vasculopathy, which lead to percutaneous coronary interventions in 1996, 1997 and 2003. An left ventricular ejection fraction (LVEF) remained normal until 2003, but progressive systolic impairment was seen in the years thereafter. In 2010, at the age of 76, echocardiography revealed an LVEF of <25% and aortic valve disease with low-gradient severe Aortic valve stenosis (aortic valve area of 1.0 cm², peak/mean gradient 32/17 mmHg) and moderate aortic regurgitation (AR). The patient complained of reduced exercise capacity, angina and dyspnoea. Coronary angiography showed diffuse transplant vasculopathy with a 90% stenosis of the posterior descending artery of the right coronary artery. The first diagonal branch was occluded, but was perfused via collaterals. Both lesions were not suitable for revascularization.
Because the true low-gradient severe aortic valve stenosis could not be confirmed by dobutamine stress echocardiography nor bicycle stress echocardiography, a percutaneous transluminal aortic valvuloplasty was performed in April 2011 to evaluate the effect on symptoms and LVEF. The LVEF improved up to 30% despite an increase in AR, and angina symptoms also improved. The 6-min walk test distance increased by 108 m, 4 months after the procedure.
Seven months later, angina symptoms relapsed, most likely due to restenosis of the aortic valve (aortic valve area of 0.9 cm², Fig. 1). The transient improvement with percutaneous transluminal aortic valvuloplasty justified AVR. The comorbidities and condition of the patient, however, resulted in a prohibitive perioperative risk for surgical AVR (EuroSCORE II showed of 26.74%, too high risk for surgery), and the patient was scheduled for TAVI. As computed tomography revealed diffuse calcifications and an abdominal aneurysm with an intraluminal thrombus, a transapical approach was proposed.
Figure 1:
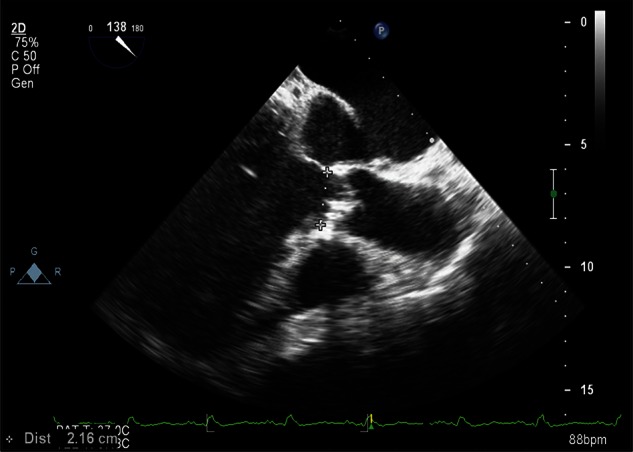
A cross-sectional two-dimensional image with the calcified cusps and diameter of the aortic annulus.
The patient was operated on under general anaesthesia in a hybrid room with transoesophageal echocardiography (TOE) monitoring. A pigtail for arteriography was inserted through the left femoral artery. Through an anterolateral mini-thoracotomy (fifth intercostal space), the apex was visualized and an epicardial ventricular pacemaker lead placed. Percutaneous transluminal aortic valvuloplasty was performed (a 20-mm balloon) during rapid ventricular pacing through a 14-Fr sheath inserted through an apical purse string. After exchange for the Ascendra introducer, a 26-mm Edwards-Sapien pericardial valve (Edwards Lifesciences, Irvine, CA, USA) was positioned at the level of the native aortic valve (Fig. 2A). The valve was deployed during rapid ventricular pacing and ventilatory arrest. An aortogram confirmed good valve position and patent coronary arteries (Fig. 2B and C). TOE showed a minimal paravalvular leakage. The patient was extubated in the operation theatre and received initial care in the coronary care unit for 3 days. Renal function recovered to a preoperative value (2.21 mg/dl), and he was discharged on postoperative day 6.
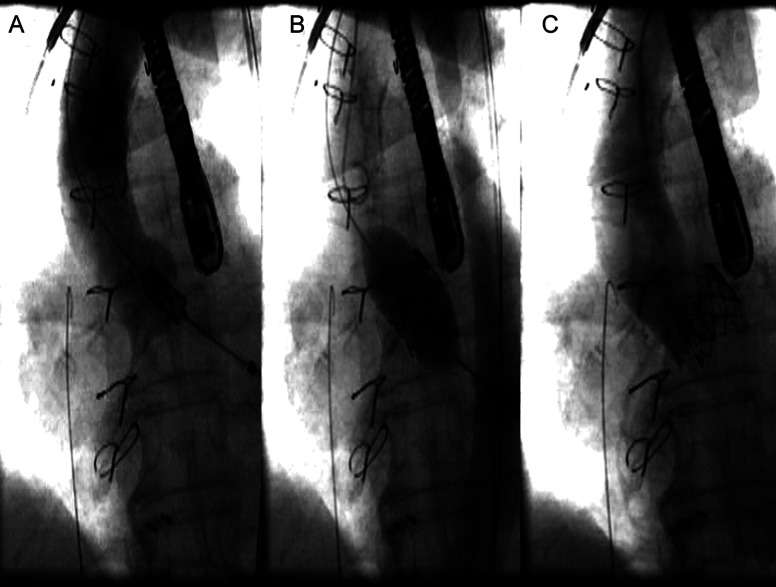
Figure 2:
(A) Positioning of the aortic valve according to the aortogram. The prosthetic valve is positioned symmetrically over the aortic annulus (B). Expanding the valve during rapid pacing (C). The valve in the correct position. Minimal aortic regurgitation and normal filling of both coronary arteries.
Re-evaluation 6 weeks after TAVI demonstrated a stable LVEF of 30% and properly functioning valve (aortic valve area = 2.4 cm2; gradients: 18/11 mmHg; no AR). Six-min walking distance was comparable with the test early after percutaneous transluminal aortic valvuloplasty (482 vs 491 m). Six months after TAVI, LVEF increased to 35% with preserved valve prosthesis function (aortic valve area = 2.5 cm2; gradients: 11/7 mmHg), and the patient was in good clinical condition.
DISCUSSION
TAVI has been rarely performed in heart transplant patients. The prevalence of Aortic valve stenosis in transplant patients will probably increase over the next years due to the longer survival of heart transplant recipients and less strict donor criteria [4].
The modest improvement in EF (25–35%) after TAVI can be explained by the long-standing underlying ischaemic cardiomyopathy, which allowed only partial functional ventricular recovery. Improvement in valve function did, however, significantly reduce the afterload, resulting in improved clinical performance and freedom from angina.
To our knowledge, this is only the third case reporting TAVI in heart transplant recipients, and the first in a patient with the severely depressed LVEF [4, 5]. The risk score of this patient was prohibitive for surgical AVR. Indeed, in this case, conventional surgery had to cope with a second redo setting, calcification of ascending aorta, renal failure, peripheral vascular disease and poor wound healing due to immunosuppressive treatment. A transapical approach in this patient seemed to be optimal due to a significant aortic and peripheral vascular disease. TAVI confirms to be a feasible technique in heart transplant recipients with multiple risk factors.
Conflict of interest: none declared.
REFERENCES
- 1.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 2.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 3.Benden C, Aurora P, Edwards LB, Kucheryavaya AY, Christie JD, Dobbels F, et al. The Registry of the International Society for Heart and Lung Transplantation: Fourteenth Pediatric Lung and Heart-Lung Transplantation Report–2011. J Heart Lung Transplant. 2011;30:1123–32. doi: 10.1016/j.healun.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Seiffert M, Meyer S, Franzen O, Conradi L, Baldus S, Schirmer J, et al. Transcatheter aortic valve implantation in a heart transplant recipient: a case report. Transplant Proc. 2010;42:4661–3. doi: 10.1016/j.transproceed.2010.09.148. [DOI] [PubMed] [Google Scholar]
- 5.Bruschi G, De Marco F, Oreglia J, Colombo P, Moreo A, De Chiara B, et al. Transcatheter aortic valve implantation after heart transplantation. Ann Thorac Surg. 2010;90:e66–8. doi: 10.1016/j.athoracsur.2010.08.021. [DOI] [PubMed] [Google Scholar]