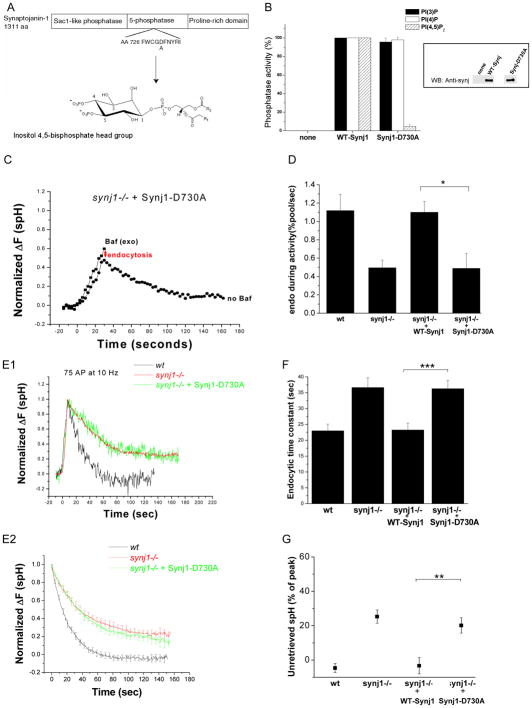
Figure 5. The 5-phosphatase activity of Synj1 controls both the endocytic rate during persistent activity and endocytosis for small stimuli.
(A) Design of the 5-phosphatase activity deficient mutant (Synj1-D730A). An aspartate residue conserved in all mammalian inositol-5-phosphatases and previously shown to be important for catalytic activity (Whisstock et al., 2000; Whisstock et al., 2002) was mutated to alanine.
(B) The D730A mutation inhibits 5-phosphatase activity of Synj1. Flag-tagged Synj1 (WT or D730A) was incubated with the phosphoinositide substrate, and the free phosphate generated was measured using the Malachite green phosphatase assay (Harder et al., 1994; Lee, et al, 2004). Phosphatase activities are normalized with wildtype Synj1 set to 100%. Mean and SEM of 3–4 expts are presented. (Inset) Equal amounts of immunoprecipitated Synj was tested by Western blotting with the polyclonal α-Synj1 antibody.
(C) Representative example of the response to a 10 Hz, 30 sec stimulus. The red arrow indicates the extent of endocytosis during activity.
(D) The endocytic rate during activity is reduced in Synj1-D730A-expressing neurons as compared to those expressing WT-Synj1. *P<0.01, n=8 for Synj1-D730A. Error bars are SEM.
(E1, 2) Endocytosis after short stimuli is not restored by expression of Synj1-D730A. Representative examples of endocytosis after 75 AP (E1), and the average endocytic time course for 50–100 AP (E2) in wt (black), synj1−/− (red), and synj1−/−+Synj1-D730A (green) conditions.
(F) The endocytic time constant for 50–100 stimuli is not restored by the expression of Synj1-D730A. ***P<0.001.
(G) The percentage of unretrieved spH remains elevated despite expression of Synj1-D730A. **P<0.002
n=14 for Synj1-D730A. Error bars are SEM.