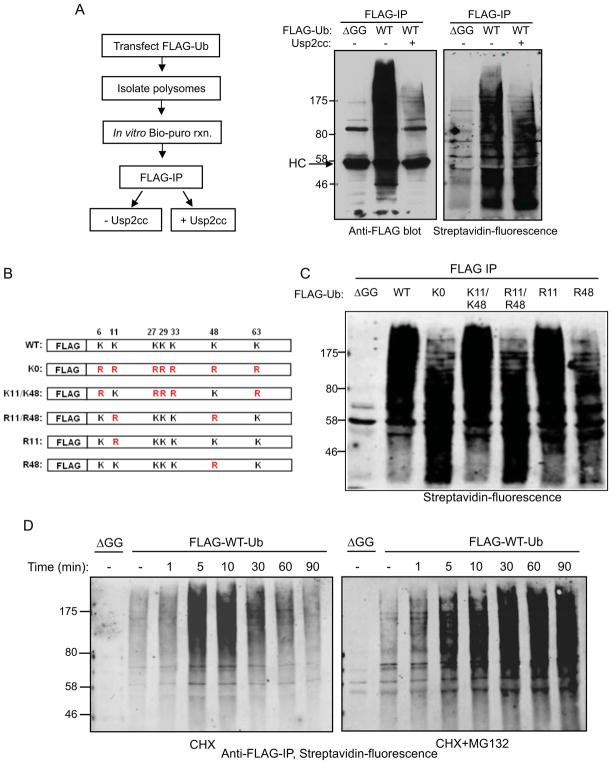
Figure 3. CTU products contain primarily K48-linked polyubiquitin chains.
A. CTU target proteins are multubiquitinated. Ubiquitinated nascent polypepetides were labeled with Bio-Puro, and immunoprecipitated with anti-FLAG antibody. The immunoprecipitated products were treated with the catalytic domain of ubiquitin-specific protease 2 (USP2cc) to strip ubiquitin (see schematic, left). Middle panel is the anti-FLAG-Ub blot analysis of samples before and after USP2cc treatment (HC is IgG heavy chain). Right panel shows the fluorescent-streptavidin blot of the FLAG IPs. The molecular weight change of CTU products after USP2cc treatment indicates that the majority of the CTU products contained multiple ubiquitin moieties. B. Schematic diagram of mutant forms of FLAG-ubiquitin expressed in HEK293T cells. C. Polysomes were isolated from cells expressing the indicated form of ubiquitin. Nascent chains were labeled with Bio-Puro in vitro, and then immunoprecipitated with anti-FLAG antibody. The immunoprecipitated products were subject to SDS-PAGE, and detection was with fluorescent streptavidin. All exogenously expressed ubiquitin mutants were expressed at similar levels (not shown). D. HEK293T cells were transfected with plasmids expressing FLAG-Ub or FLAG-Ub-ΔGG. Prior to lysis, cells were treated with CHX alone or simultaneously with CHX and MG132 for the indicated times. Polysomes were isolated from these cells and reacted with Bio-Puro. Nascent polypeptides were analyzed by anti-FLAG immunoprecipitation and detection with fluorescent streptavidin. See also Figure S3.