Abstract
We assessed laboratory monitoring following combination antiretroviral therapy (cART) initiation among 3,678 patients in a large US multi-site clinical cohort, censoring participants at last clinic visit, cART change, or three years. Median days (interquartile range) to first hematologic, hepatic, renal and lipid tests were 30 (18–53), 31 (19–56), 33 (20–59) and 350 (96–1106), respectively. At one year, approximately 80% received more than two hematologic, hepatic, and renal tests consistent with guidelines. However, only 40% received one or more lipid tests. Monitoring was more frequent in specific subgroups, likely reflecting better clinic attendance or clinician perception of higher susceptibility to toxicities.
Keywords: Laboratory Monitoring, Antiretroviral Therapy, Antiretroviral Toxicity
Introduction
Combination antiretroviral therapy (cART) is associated with adverse effects of variable severity. Adverse effects are among the most common reasons for antiretroviral agent changes and may be associated with end-organ damage and higher mortality1–3. While symptomatic effects prompt clinical intervention, asymptomatic adverse effects may go undetected and result in cumulative toxicity. Laboratory monitoring during cART is endorsed in developed countries4,5, but guidelines are flexible for resource-limited settings6 due in part to data suggesting that cART can be delivered safely without routine laboratory monitoring7.
The United States Department of Health and Human Services (DHHS) recommends laboratory monitoring throughout the course of cART4. This includes serum alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin, creatinine, and complete blood count 2–8 weeks after initiating or modifying cART. A repeat of these tests is recommended every 3–6 months thereafter. Fasting lipid profile is recommended at least annually4.
Actual laboratory monitoring practices during cART in routine clinical care in the U.S are unknown. This information is critical to evaluate resource utilization and assess adherence to guidelines. We evaluated laboratory monitoring among patients who initiated their first cART regimen between 2000–2010 in a multi-site US clinical cohort.
Methods
This observational clinical cohort included antiretroviral-naïve patients participating in the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) who initiated a first modern cART regimen between 2000–2010 and had at least one laboratory test of any type after cART initiation. We defined modern cART as a regimen containing a ritonavir-boosted protease inhibitor (PI), non-nucleoside reverse transcriptase inhibitor (NNRTI), or integrase inhibitor plus ≥2 nucleoside/nucleotide reverse transcriptase inhibitors (NRTI). Patients were excluded if their regimen included stavudine, didanosine or unboosted-PIs, or if they had an abnormal value for the laboratory test being investigated before cART initiation. CNICS is a collaboration of eight US HIV clinical cohorts8. Ethical approval was obtained for CNICS from each site’s Institutional Review Board.
We analyzed hematologic, hepatic, renal, and lipid indices following cART initiation. Hematologic measures included hemoglobin, absolute neutrophil count or platelet count. Hepatic measures included ALT or AST. We assessed creatinine and non-HDL cholesterol (calculated by subtracting HDL from total cholesterol) for renal and lipid assessments, respectively. Abnormal values were defined as: hematologic= hemoglobin≤10 g/dL, neutrophil count≤750 cells/mm3, or platelet count≤50×109/L; hepatic= ALT or AST≥3 times the upper limit of normal; renal= estimated glomerular filtration rate<50mL/min/1.73m2; and lipid= non-HDL cholesterol≥160 mg/dL. All analyses were stratified by laboratory category.
Patients contributed time from cART initiation to the first of: switch/discontinuation of any antiretroviral for >14 days, loss-to-follow-up (>12 months without a clinic visit), last clinic visit before Dec. 31, 2010, or 3 years post-cART initiation because <15% of patients remained. Post-cART initiation, if an abnormality occurred for the laboratory category being assessed, patient-time was censored immediately after the abnormality (e.g., patient with hepatic abnormality was censored when assessing hepatic monitoring but not other laboratory indices). The initial abnormal test was counted as an event.
We calculated median time-to-first-test, and the cumulative number of tests through 3, 6, 12, 18, and 24 months of cART use. Poisson regression was used to estimate overall testing rates and rates by time interval following cART initiation. Bivariable and multivariable Cox regression was used to estimate hazard ratios and 95% confidence intervals in order to evaluate demographic and clinical characteristics at cART initiation as predictors of time-to-first-laboratory-test. Repeated events analyses were conducted to evaluate predictors of time-to-laboratory-test incorporating all tests an individual had during follow-up, using the Anderson-Gill model and a robust covariance estimator9. Multivariable models included study site and all variables predictive in bivariable analysis (P<0.10). All analyses were conducted in SAS10.
Results
Between 2000–2010, 3678 patients started their initial cART regimen and had available follow-up laboratory data. We excluded patients (N) with hematologic (91), hepatic (98), renal (129), and lipid (200) abnormalities pre-cART from each respective analysis. No patients were excluded from all analyses as no patient had all four abnormalities. Overall, 82% were male, 43% white, 37% Black, 15% Hispanic, and 5% other race; 47% were men who have sex with men; and 8% reported injection drug use. At cART initiation, median age was 39 years (interquartile range [IQR]:32–45), median year was 2006 (IQR:2004–2008), median CD4 cell count was 204 cells/mm3 (IQR:64–315) and the median HIV RNA level was 4.90 log10 copies/ml (IQR:4.41–5.39). Five percent were hepatitis B co-infected and 15% were hepatitis C co-infected. Initial cART regimens were predominantly NNRTI-based (63%), of which 93% included efavirenz. Additionally, 37% were boosted-PI-based; of these 56% included atazanavir and 35% lopinavir/ritonavir. Less than 1% of regimens were integrase-inhibitor-based. Most common NRTIs were emitricitabine/lamivudine (100%), tenofovir (72%), zidovudine (22%), and abacavir (10%).
A switch or discontinuation of an antiretroviral drug led to censoring of 39% of patients (N=1434), with a median time to switch/discontinuation of 8.1 months (IQR:3.0–20.2). Additionally, 26% of patients were lost-to-follow-up, and 21% were censored on December 31, 2010. By 3 years post-cART initiation, 14% of patients (N=515) remained in the study. Overall, median follow-up time was 11.1 months (IQR:3.8–24.1) with total follow-up of 5112 person-years. For hematologic, hepatic, renal, and lipid analyses, 451, 338, 114, and 553 patients, respectively, were censored earlier because of an abnormal test.
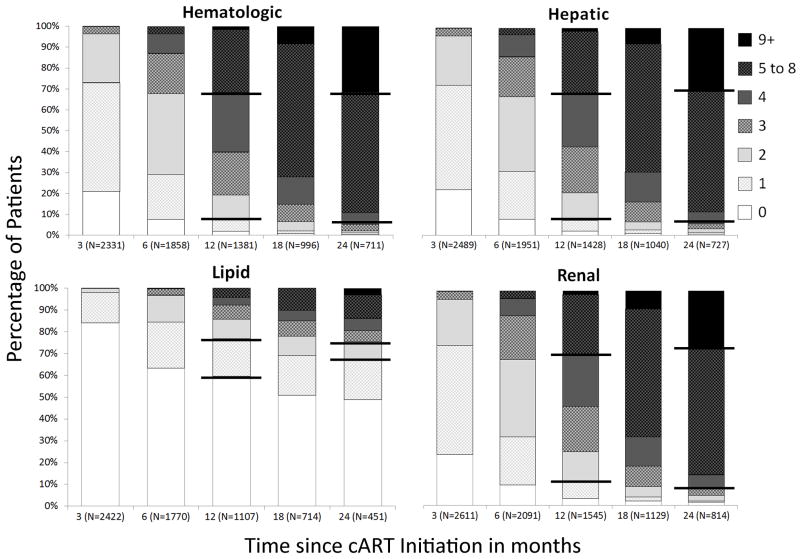
Median days (IQR) to first laboratory test were 30 (18–53), 31 (19–56), 33 (20–59) and 350 (96–1106) for hematologic, hepatic, renal and lipid, respectively. In the first 3 months of cART, most patients received ≥1 hematologic, hepatic and renal laboratory measure (79%, 78%, and 76%, respectively) and very few a lipid test (16%) (Figure 1). Among patients still in follow-up one year post-cART initiation, 81%, 79%, and 78% had obtained tests on ≥2 occasions for hematologic, hepatic, and renal, respectively, while 40% received ≥1 lipid test. Among patients in follow-up for two years, 51% received ≥1 lipid tests.
Figure 1.
Cumulative number of tests by laboratory category across time since combination antiretroviral therapy (cART) initiation, CFAR Network of Integrated Clinical Systems, between 2000 and 2010. *
*Bold horizontal lines represent range for expected number of laboratory tests based on 2010 DHHS guidelines at 1 year and at 2 years. These ranges were calculated by taking the recommended frequency of testing and calculating the minimum and maximum number of tests recommended during the time interval. For example, the minimum for hematologic, hepatic, and renal tests at one year was calculated as one test for 8 weeks post-cART initiation and one test 6 months later for a total for a total of 2 tests. Similarly, the maximum was calculated as one test 2 weeks post-cART and then one test for each 3 month interval thereafter, totaling 4 tests within the first year.
Monitoring for hematologic, hepatic, and renal tests was highest in the first 6 months of cART (p<0.001) and plateaued at a lower rate thereafter. Testing rates during the first 6 months of cART were 1.46, 1.42, and 1.37 per 100 person-days for hematologic, hepatic, and renal, respectively. The corresponding rates between months 6 and 36 of cART were 0.97, 0.93, and 0.92 per 100 person-days. In contrast, lipid monitoring was consistent across time from cART initiation and less frequent than other tests at 0.30 per 100 person-days.
We assessed predictors of the rate of first testing and the rate of testing across follow-up time, stratified by laboratory category (Table 1). More recent calendar years of cART initiation were associated with shorter times to first lipid test as well as greater lipid monitoring rates during follow-up. Conversely, the frequency of hematologic, hepatic, and renal testing appeared stable throughout calendar time. Lower CD4 count and AIDS diagnosis prior to cART were consistently associated with shorter time to all laboratory tests and higher monitoring rates across time for all tests except lipids. For hematologic, hepatic, and renal tests, older age also predicted higher rates of first testing and testing across time. Hepatitis C co-infection was predictive of higher rates for repeated hematologic tests, but was not predictive for other monitoring.
Table 1.
Independent predictors of laboratory monitoring following combination antiretroviral therapy initiation by laboratory category, CFAR Network of Integrated Clinical Systems, between 2000 and 2010.
| First Laboratory Test* | Repeated Tests* | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Hematologic Tests | ||
| Age (by decade increase) | 1.06 (1.03, 1.10) | 1.03 (1.00, 1.05) |
| Female Sex | - | 1.02 (0.95, 1.09) |
| MSM | - | 1.01 (0.96, 1.06) |
| IDU | 0.73 (0.64, 0.84) | - |
| CD4 count (by 100 cells/mm3 increase) | 0.94 (0.92, 0.97) | 0.99 (0.97, 1.01) |
| Viral load (by log10 increase) | 1.01 (0.96, 1.07) | 1.00 (0.96, 1.03) |
| AIDS Diagnosis | 1.28 (1.18, 1.39) | 1.24 (1.16, 1.31) |
| cART start year | 1.00 (0.99, 1.02) | 1.00 (0.99, 1.02) |
| Boosted-PI (compared to NNRTI) | 1.18 (1.10, 1.26) | 1.12 (1.07, 1.17) |
| Zidovudine Use | - | 1.10 (0.97, 1.25) |
| Tenofovir Use | 1.01 (0.91, 1.13) | 1.05 (0.93, 1.19) |
| Abacavir Use | 1.32 (1.16, 1.51) | 1.27 (1.14, 1.42) |
| Hepatitis C | - | 1.13 (1.05, 1.22) |
| Hypertension | - | 1.11 (1.01, 1.21) |
| Hyperglycemia | 1.13 (0.99, 1.30) | 1.04 (0.99, 1.10) |
| Hepatic Tests | ||
| Age (by decade increase) | 1.07 (1.03, 1.11) | 1.04 (1.01, 1.06) |
| Female Sex | - | 1.07 (0.99, 1.15) |
| Race/Ethnicity | ||
| White | Ref. | Ref. |
| Black | 0.92 (0.85, 1.00) | 0.93 (0.88, 0.98) |
| Other | 1.06 (0.97, 1.16) | 1.05 (0.98, 1.13) |
| MSM | 1.12 (1.04, 1.21) | 1.02 (0.97, 1.07) |
| IDU | 0.85 (0.73, 0.98) | - |
| CD4 count (by 100 cells/mm3 increase) | 0.95 (0.92, 0.97) | 0.97 (0.96, 0.98) |
| Viral load (by log10 increase) | 0.97 (0.92, 1.02) | 0.99 (0.95, 1.02) |
| AIDS Diagnosis | 1.33 (1.22, 1.44) | 1.22 (1.15, 1.30) |
| cART start year | - | 1.01 (1.00, 1.02) |
| Boosted-PI (compared to NNRTI) | 1.23 (1.14, 1.32) | 1.15 (1.10, 1.21) |
| Zidovudine Use | 1.16 (0.94, 1.43) | - |
| Tenofovir Use | 1.38 (1.10, 1.71) | 1.00 (0.93, 1.08) |
| Abacavir Use | 1.53 (1.28, 1.82) | 1.26 (1.16, 1.37) |
| Hepatitis B | 1.10 (0.95, 1.29) | - |
| Hepatitis C | 0.95 (0.85, 1.05) | 1.02 (0.93, 1.12) |
| Hypertension | - | 1.09 (1.00, 1.18) |
| Hyperglycemia | 1.19 (1.03, 1.36) | 1.03 (0.98, 1.09) |
| Renal Tests | ||
| Age (by decade increase) | 1.06 (1.03, 1.10) | 1.04 (1.01, 1.07) |
| Female Sex | - | 1.02 (0.92, 1.13) |
| Race | ||
| White | Ref. | |
| Black | - | 0.95 (0.88, 1.03) |
| Other | - | 1.07 (0.97, 1.18) |
| MSM | - | 0.96 (0.89, 1.04) |
| IDU | 0.77 (0.67, 0.88) | - |
| CD4 count (by 100 cells/mm3 increase) | 0.95 (0.93, 0.97) | 0.95 (0.93, 0.97) |
| Viral load (by log10 increase) | 0.98 (0.93, 1.03) | 0.96 (0.91, 1.01) |
| AIDS Diagnosis | 1.29 (1.19, 1.40) | 1.37 (1.27, 1.49) |
| cART start year | 1.01 (1.00, 1.03) | 1.01 (1.00, 1.03) |
| Boosted-PI (compared to NNRTI) | 1.13 (1.05, 1.22) | 1.14 (1.06, 1.22) |
| Zidovudine Use | 1.14 (0.92, 1.42) | - |
| Tenofovir Use | 1.41 (1.13, 1.75) | 1.01 (0.91, 1.13) |
| Abacavir Use | 1.58 (1.32, 1.89) | 1.21 (1.07, 1.36) |
| Hepatitis C | - | 1.03 (0.93, 1.14) |
| Hypertension | 1.06 (0.97, 1.15) | 1.08 (1.01, 1.16) |
| Hyperglycemia | 1.21 (1.05, 1.39) | 1.20 (1.04, 1.39) |
| Lipid Tests | ||
| Age (by decade increase) | 1.02 (0.97, 1.07) | 1.02 (0.98, 1.06) |
| Race/Ethnicity | ||
| White | Ref. | Ref. |
| Black | 1.09 (0.98, 1.21) | 0.90 (0.82, 0.99) |
| Other | 0.88 (0.77, 1.01) | 0.92 (0.83, 1.03) |
| IDU | - | 1.01 (0.84, 1.21) |
| CD4 count (per 100 cells/mm3 increase) | 0.95 (0.93, 0.98) | - |
| AIDS Diagnosis | 1.14 (1.02, 1.28) | - |
| cART start year | 1.06 (1.04, 1.09) | 1.02 (1.00, 1.05) |
| Zidovudine Use | 1.09 (0.93, 1.28) | - |
| Tenofovir Use | - | 1.07 (0.94, 1.23) |
| Abacavir Use | 1.21 (1.03, 1.43) | 1.52 (1.30, 1.77) |
| Hepatitis C | - | 0.94 (0.83, 1.07) |
| Hypertension | 1.14 (1.02, 1.29) | - |
| Hyperglycemia | 1.22(1.00, 1.47) | 1.11 (0.96, 1.27) |
multivariable regression includes all variables predictive in bivariable analysis (all variables with results in column) as well as study site.
HR=hazard ratio; MSM=men who have sex with men; IDU=injection drug user; cART=combination antiretroviral therapy; PI=protease inhibitor; NNRTI=non-nucleoside reverse transcriptase inhibitor
Abacavir use was associated with shorter time to first testing and increased monitoring rates across follow-up for all laboratory types. For hematologic, hepatic, and renal monitoring, patients on boosted-PI regimens had shorter times to first testing and higher monitoring rates during follow-up compared to patients with NNRTI-based regimens. Tenofovir use was associated with shorter time to first hepatic and renal tests, but did not appear predictive of monitoring rates for repeated tests across time.
Hyperglycemia (diabetes mellitus diagnosis or blood glucose measure >120mg/dL prior to cART) was predictive of shorter time to first hepatic, renal, and lipid testing, but was only significantly associated with higher renal monitoring rates across follow-up. Hypertension (diagnosis or blood pressure >140/90 on at least 2 occasions >1 month apart prior to cART) was associated with shorter time to first lipid test, but was not predictive of lipid monitoring rates thereafter. Conversely, while hypertension was not significantly associated with time to first hematologic, hepatic, or renal tests, it was predictive of overall higher monitoring rates for these tests across time.
Discussion
In this clinical HIV cohort, routine laboratory monitoring was as frequent as recommended for hematologic, hepatic, and renal tests for the majority of patients initiating cART between 2000–2010, but lipid monitoring was substantially less than recommended4. Specific clinical characteristics predicted the relative frequency of testing. This study evaluated a national multi-site population of HIV-infected patients initiating modern cART in routine clinical care, allowing our findings to be broadly generalizable in the U.S.
Over 75% of patients received the minimum number of recommended tests for hematologic, hepatic, and renal abnormalities by one year of cART. More than 30% received more tests than recommended by one year. Because we only excluded patients based on prior abnormalities for each laboratory type, these extra tests were likely driven by preceding diagnoses, co-administration of other medications, symptoms, or abnormalities on other laboratory tests. In contrast, most patients did not have an annual lipid evaluation, and only 20% of performed tests occurred in the recommended fasting state. In fact, less than half of patients received a lipid test within the first year, and among patients followed for two years, only 51% received a lipid test. This is similar to results from a previous study within CNICS, where 59% of patients had at least one non-HDL measurement during a mean 1.7 years of antiretroviral therapy11. As other monitoring tests were obtained more frequently, it is unlikely that poor lipid monitoring was driven by poor visit attendance. An alternative explanation is that clinicians were hesitant to perform lipid testing in patients who were not fasting. Monitoring for lipid abnormalities appeared to improve in more recent years, but it remains substandard.
We observed patterns suggesting that clinicians were selective in the abnormalities monitored among patient subgroups. Specifically, hematologic, hepatic, and renal tests were obtained more frequently in those with older age, more advanced HIV disease, comorbidities, or boosted-PI use. As these tests are often obtained together, it is unsurprising that a predictor for receiving one type of test may lead to more frequent testing for all three laboratory categories. While these predictive characteristics may represent patients with better clinic attendance12,13, these observations likely reflect increased clinician vigilance in subgroups perceived to have higher susceptibility to toxicities14–16. Consistent with this, lipids were monitored more frequently in patients with hypertension and hyperglycemia. Similarly, the association of abacavir use with lipid and renal monitoring may reflect both the tendency to channel patients with possible renal risk to abacavir, and concerns that abacavir increases cardiovascular risk, though this is controversial17.
Study limitations include the assessment of monitoring rates only among patients on their first cART without prior abnormal laboratory values; therefore findings are not generalizable to patients on subsequent cART regimens or patients with documented laboratory abnormalities. This approach also resulted in a high-rate of censoring, reflective of regimen changes and laboratory abnormalities in routine clinical practice18–20. While current DHHS guidelines specifically recommend fasting lipid panel4, we considered both fasting and random non-HDL measurements in our analysis. Restriction to the recommended fasting lipids would have resulted in substantially lower estimates of compliance with lipid monitoring. While our estimates may underestimate all lipid monitoring, isolated total cholesterol values provide incomplete screening for lipid abnormalities, particularly among HIV patients who often do not exhibit classic dyslipidemic patterns. Also, patients may have obtained tests outside of the CNICS clinics, potentially leading to underestimates of monitoring rates, though this is unlikely to explain the discrepancy between monitoring of lipids and other standard labs.
In conclusion, most patients received frequent monitoring in accordance with current guidelines4 for hematologic, hepatic, and renal abnormalities, but not lipid abnormalities. Clinicians may be tailoring laboratory monitoring based on perceived risks, and therefore further assessments of adverse events among patients initiating cART in routine clinical care are needed. Future research should also focus on optimizing laboratory monitoring strategies in resource-rich settings, recognizing that research in resource-poor settings has challenged the benefit and cost-effectiveness of asymptomatic laboratory screening in all patient populations7,21.
Acknowledgments
This study was supported by grants R24-AI067039, P30-AI50410, and 5T32-AI007001-35 from the National Institutes of Health, R01-HS018731 from the Agency for Healthcare Research and Quality. The funding sources did not participate in the study design; collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the paper for publication. We would like to thank the patients, principal investigators, co-investigators, and research staff at participating CFAR Network of Integrated Clinical Systems sites at the following institutions: Case Western Reserve University; University of Alabama at Birmingham; University of California at San Francisco; University of Washington; University of California at San Diego; Fenway Community Health Center of Harvard University; University of North Carolina at Chapel Hill; and Johns Hopkins University. In particular, we thank Stephen Van Rompaey of the Data Management Core at University of Washington, Donna Porter of the Administrative Core at University of Alabama at Birmingham, and Benigno Rodriguez and Michael Lederman at Case Western Reserve University for their assistance in the conduct of this study.
Footnotes
Previous Presentation: Portions of the data were previously presented at IDWeek, October 17-21, 2012 in San Diego, CA, USA.
Authors and Contributions
ELY, BT and SN led the conception and design of this study, contributed to data acquisition and interpretation, prepared the initial draft of the manuscript, had full access to all the data in the study and take final responsibility for the decision to submit for publication. JJE, PR and SLK substantively contributed to the conception and design of this study, data acquisition and/or interpretation and provided critical revision of the manuscript. Remaining authors provided substantial contributions to the study design, data acquisition and/or interpretation of data and provided critical revision of the manuscript. All authors approved the final version of the manuscript.
Conflicts of Interest: SN has received grant support from Pfizer, Bristol-Myers Squibb and Merck. B.T. has served as an advisor and/or received research support (to Northwestern University) from Janssen, GlaxoSmithKline and ViiV. J.J.E is a consultant to Bristol Myers Squibb, GlaxoSmithKline, Merck, ViiV and Janssen, and has received research support (to UNC) from GlaxoSmithKline, Bristol Myers Squibb and Merck. All remaining authors have no conflicts of interest to declare.
References
- 1.Elzi L, Marzolini C, Furrer H, et al. Treatment modification in human immunodeficiency virus-infected individuals starting combination antiretroviral therapy between 2005 and 2008. Arch Intern Med. 2010;170(1):57–65. doi: 10.1001/archinternmed.2009.432. [DOI] [PubMed] [Google Scholar]
- 2.Keiser O, Fellay J, Opravil M, et al. Adverse events to antiretrovirals in the Swiss HIV Cohort Study: effect on mortality and treatment modification. Antiviral Therapy. 2007;12(8):1157–1164. [PubMed] [Google Scholar]
- 3.Neuman MG, Schneider M, Nanau RM, Parry C. HIV-antiretroviral therapy induced liver, gastrointestinal, and pancreatic Injury. Int J Hepatology. 2012 doi: 10.1155/2012/760706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents; United States Department of Health and Human Services, editor. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Office of AIDS Research Advisory Council; 2012. http://www.aidsinfo.nih.gov/guidelines. [Google Scholar]
- 5.Clumeck N, Pozniak A, Raffi F, Committee EE. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of HIV-infected adults. HIV Medicine. 2008;9:65–71. doi: 10.1111/j.1468-1293.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. World Health Organization; 2010. http://www.who.int/hiv/pub/arv/adult2010/en/index.html. [PubMed] [Google Scholar]
- 7.Mugyenyi P, Walker AS, Hakim J, et al. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet. 2010;375(9709):123–131. doi: 10.1016/S0140-6736(09)62067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. International journal of epidemiology Oct. 2008;37(5):948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. Journal of the American Statistical Association. 1989;84(408):1074–1078. [Google Scholar]
- 10.SAS version 9.2 [computer program] Cary, NC, USA: 2011. [Google Scholar]
- 11.Crane HM, Grunfeld C, Willig JH, et al. Impact of NRTIs on lipid levels among a large HIV-infected cohort initiating antiretroviral therapy in clinical care. AIDS. 2011 Jan 14;25(2):185–195. doi: 10.1097/QAD.0b013e328341f925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mugavero MJ, Lin HY, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009 Jan 15;48(2):248–256. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulett KB, Willig JH, Lin H, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS patient care and STDs. 2009;23(1):41–49. doi: 10.1089/apc.2008.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Santis GC, Brunetta DM, Vilar FC, et al. Hematological abnormalities in HIV-infected patients. International Journal of Infectious Diseases. 2011;15:e808–e811. doi: 10.1016/j.ijid.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Sulkowski MS. Drug-induced liver injury associated with antiretroviral therapy that includes HIV-1 protease inhibitors. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;38(Supplement 2):S90–S97. doi: 10.1086/381444. [DOI] [PubMed] [Google Scholar]
- 16.Overton ET, Nurutdinova D, Freeman J, Seyfried W, Mondy KE. Factors associated with renal dysfunction within an urban HIV-infected cohort in the era of highly active antiretroviral therapy. HIV Med. 2009;10(6):343–350. doi: 10.1111/j.1468-1293.2009.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruciani M, Zanichelli V, Serpelloni G, et al. Abacavir use and cardiovascular disease events: a meta-analysis of published and unpublished data. AIDS. 2011;25:1993–2004. doi: 10.1097/QAD.0b013e328349c6ee. [DOI] [PubMed] [Google Scholar]
- 18.Mugavero MJ, May M, Harris R, et al. Does short-term virologic failure translate to clinical events in antiretroviral-naive patients initiating antiretroviral therapy in clinical practice? AIDS. 2008 Nov 30;22(18):2481–2492. doi: 10.1097/QAD.0b013e328318f130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The Spectrum of Engagement in HIV Care and its Relevance to Test-and-Treat Strategies for Prevention of HIV Infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taiwo B, Yanik E, Napravnik S, et al. Laboratory abnormalities following initiation of modern antiretroviral therapy (ART) in the United States, 2000–2010 among the CNICS Cohort. Conference on Retroviruses and Opportunistic Infections; Seattle, WA. 2012. [Google Scholar]
- 21.Koenig SP, Schackman BR, Riviere C, et al. Clinical impact and cost of monitoring for asymptomatic laboratory abnormalities among patients receiving antiretroviral therapy in a resource-poor setting. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010 Sep 1;51(5):600–608. doi: 10.1086/655762. [DOI] [PMC free article] [PubMed] [Google Scholar]