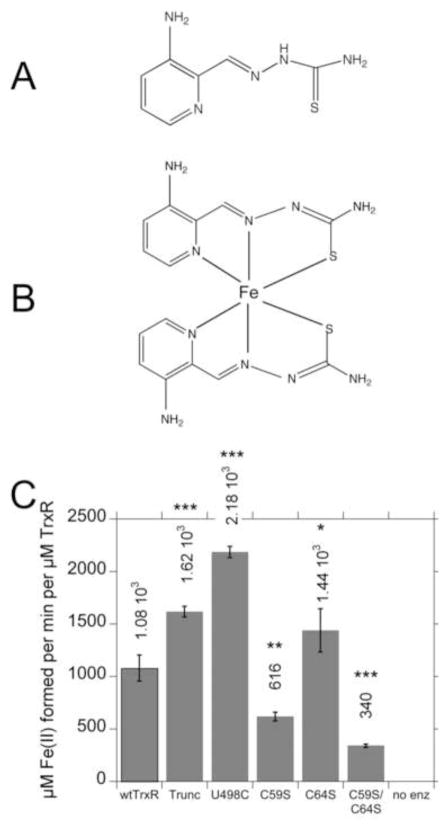
Fig. 4.
Chemical structures of Tp (A) and Fe(Tp)2 (B), and (C) rates of reduction of Fe(III)(Tp)2 by wtTrxR and its site-directed variants. In B, the Fe(Tp)2 structure is not planar as drawn, but rather the six donor atoms from the two Tp molecules ligate the Fe center in an octahedral fashion. C: Experiments were conducted in the same KCl-K Phos buffer system used for ESR, and contained 0.4 mM NADPH and 30 nM of the TrxR variants. The reactions were started by the addition of Fe(III)(Tp)2 to a final concentration of 50 μM, and the initial rates at 37°C (determined by the increase in absorbance at 610 nm) were taken from the linear portion during the first 20 s of the assay. The molar extinction coefficient of Fe(II)(Tp)2 at 610 nm (3.73 mM−1 cm−1) was used to calculate the rate per min per μM TrxR. The values shown are the mean ± S.D. for three independent experiments. In comparison to wtTrxR, *p<0.05, **p<0.01, ***p<0.001. The results for C59S are different from those for C59S/C64S (p<0.05). A representative experiment on which these rates are based is shown in Supplemental Fig. S4A.