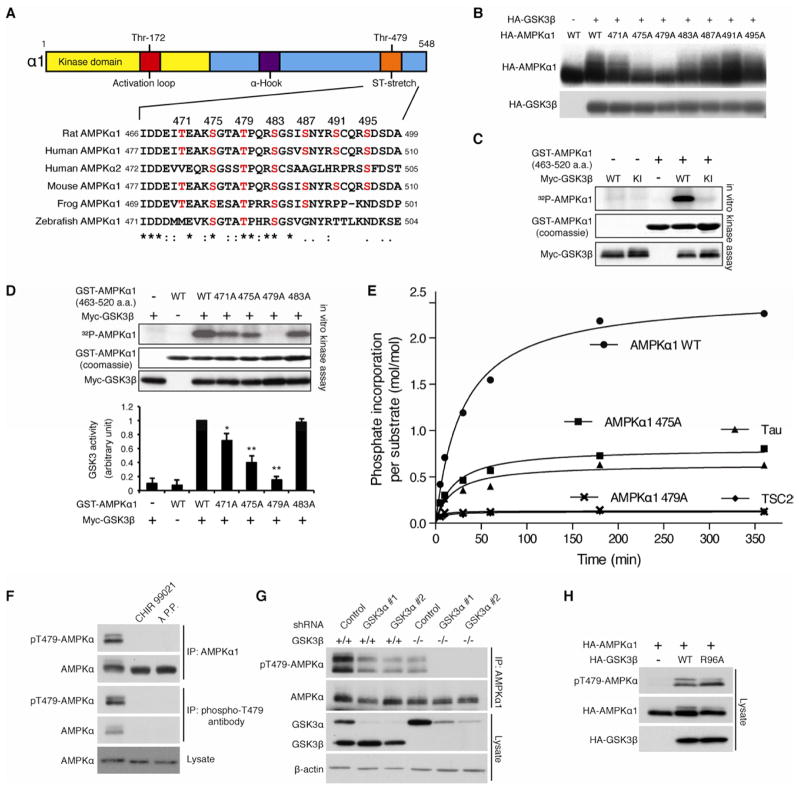
Figure 2. AMPK α subunit is an atypical substrate of GSK3.
(A) Location and sequence conservation of the ST-stretch of the αsubunit.
(B) GSK3β-induced phosphorylation of the α1 subunit is largely abolished in T479A α1 mutant in HEK293T cells. The mobility shift was monitored by Phos-Tag™ acrylamide gel electrophoresis.
(C) GSK3β phosphorylates the α1 subunit in vitro. Myc-tagged wild type or kinase inactive GSK3β was purified from HEK293T cells. A GST-AMPK α1 subunit fragment (aa. 463–520) containing the ST-stretch purified from bacteria was used as α substrate.
(D) T479A mutation of the α subunit abolishes GSK3β-induced AMPK phosphorylation in vitro. *p<0.05, **p<0.01 vs WT or 483A with GSK3, mean±SEM (n=3).
(E) Stoichiometry analysis of GSK3β-induced ST stretch phosphorylation. The indicated polypeptides (10 μM) were subjected to in vitro kinase assay using GSK3β (0.23 μM) purified from Sf21 insect cells. Note that unprimed TSC2 peptide containing GSK3 phosphorylation sites was used in this assay.
(F) GSK3 inhibitor-sensitive Thr479 phosphorylation of the α subunit. Levels of Thr479 phosphorylation of the IPed endogenous AMPK α1 subunit were detected with a phospho-specific Thr479 antibody. CHIR99021 (10 nM for 1 hr) treatment was performed.
(G) Ablation of GSK3 expression abolishes Thr479 phosphorylation of the endogenous AMPK α subunit. GSK3α was knocked down in wild or GSK3β−/− MEF cells.
(H) GSK3β mutant (R96A) phosphorylates Thr479 of the α1 subunit in HEK293T cells. See also Figures S2.