Figure 3. GSK3-induced Thr479 phosphorylation inhibits AL phosphorylation and kinase activity of the α subunit.
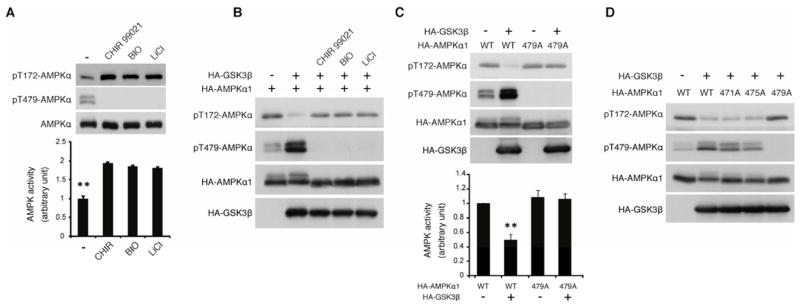
(A) Reciprocal correlation between Thr479 and Thr172 (AL) phosphorylation of the α subunit. Levels of AL and Thr479 phosphorylation and the kinase activity of endogenous AMPK α1 subunit were monitored. Pretreatment with DMSO or GSK3 inhibitors, CHIR99021 (10 nM), BIO (5 μM), or LiCl (20 mM) treatment for 1 hr was performed before harvesting the HEK293T cells. **p<0.01 vs other groups; mean±SEM (n=3).
(B) GSK3β inhibits AL phosphorylation through its kinase activity. Levels of AL and Thr479 phosphorylation were monitored in the presence or absence of GSK3 inhibitors in serum-starved HEK293T cells.
(C) GSK3β inhibits AL phosphorylation via Thr479 phosphorylation. Levels of AL and Thr479 phosphorylation and the kinase activity were monitored in serum-starved HEK293T cells. **p<0.01 vs other groups; mean±SEM (n=3).
(D) GSK3β inhibits AL phosphorylation via Thr479 but not Ser475 or Thr471 phosphorylation. GSK3β-induced reduction of AL phosphorylation in the indicated α1 mutant was monitored in serum-starved HEK293T cells. See also Figures S3.
