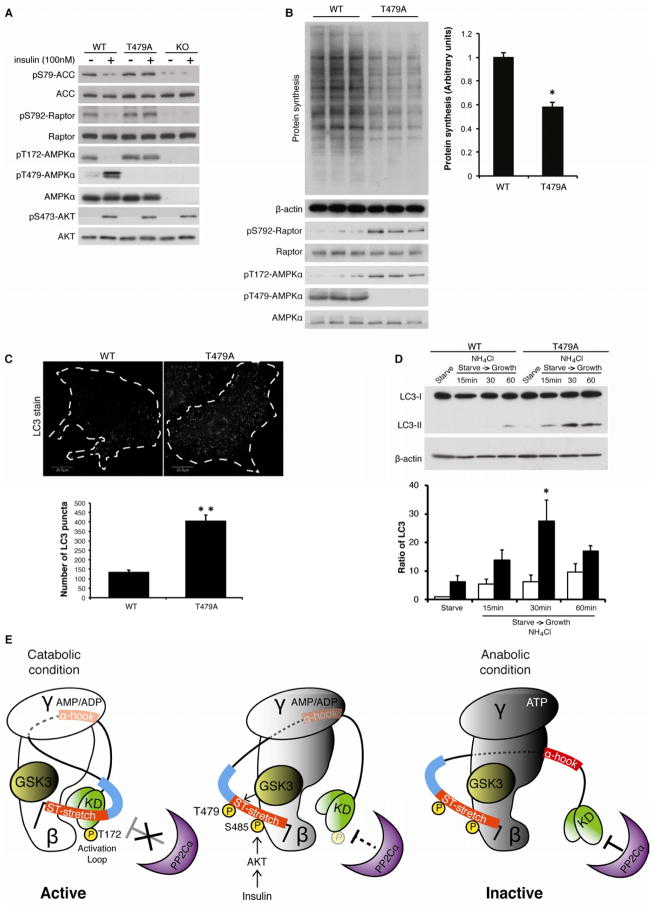
Figure 6. Loss of GSK3-dependent AMPK inhibition causes metabolic inflexibility.
(A) Insulin fails to suppress ACC1/2 and Raptor phosphorylation in T479A MEF cells. The indicated MEF cells were serum-starved for 16 hours and then stimulated with insulin (100 nM) for 60 min. See also Figures S6.
(B) Lower levels of protein synthesis are observed in T479A MEF cells. The indicated MEF cells were serum-starved for 16 hours and then stimulated with serum (10%) for 4 hours. Nascent protein synthesis was determined (upper panel). The rate of nascent protein synthesis was quantified by the expression of β-actin. *p<0.05, mean±SEM (n=3).
(C) Higher autophagic activity is observed in T479A MEF cells. Autophagosome formation was monitored in the indicated MEF cells in the presence of serum stimulation (10% for 60 min). The number of LC3-puncta per cell was measured and quantified in the indicated MEF cells. **p<0.01, mean±SEM (n=15).
(D) Higher autophagic flux is observed in T479A MEF cells. MEF cells were stimulated with serum (10% for 60 min) in the presence of NH4Cl. The ratio of LC3-II/LC3-I was quantified. *p<0.05, vs WT 30 min, mean±SEM (n=3).
(E) Schematic model of AMPK inhibition by GSK3. Under catabolic conditions (low energy and low PI3K-Akt activity), the binding of AMP or ADP to the γ subunit promotes the association of the α-hook with the γ subunit and the subsequent interaction of the kinase domain (KD) with the β subunit. Simultaneously, the non-phosphorylated ST-stretch and its adjacent region of the α subunit (blue bar) may sterically hinder phosphatase accessibility for the activation loop (left panel). In response to anabolic stimuli (high PI3K-Akt activity), successive phosphorylations on the ST-stretch of the α subunit induced by Akt and GSK3 may promote dissociation of the ST-stretch from the KD, thereby allowing the phosphatase to dephosophorylate AL loop (middle panel). Under anabolic conditions (high energy), the KD of the α subunit may be further exposed to the phosphatase through additional conformational changes in the α-hook and linker region (right panel). A previous structure study proposed that the dissociation of the α-hook from the γ subunit precedes nucleotide exchange (AMP/ADP to ATP) on the γ subunit (Xiao et al., 2011). Whether this steric change of the ST-stretch triggers dissociation of the α hook from the γ subunit remains to be resolved.