Abstract
Theta oscillations are related to cognitive functions and reflect functional integration of frontal and medial temporal structures into coherent neurocognitive networks. This study assessed event-related theta oscillations in medication-free, euthymic patients with bipolar disorder upon auditory oddball paradigm. Twenty-two DSM-IV euthymic bipolar I (n = 19) and II (n = 3) patients and twenty-two healthy subjects were included. Patients were euthymic for at least 6 months, and psychotropic-free for at least 2 weeks. EEG was recorded at 30 electrode sites. Auditory oddball paradigm and sensory stimuli were used. Event-related Oscillations were analyzed using adaptive filtering in two different theta frequency bands (4–6 Hz, 6–8 Hz). In healthy subjects, slow theta (4–6 Hz) responses were significantly higher than those of euthymic patients upon target, non-target and sensory stimuli (p < 0.05). Fast theta (6–8 Hz) responses of healthy subjects were significantly higher than those of euthymic patients upon target-only stimuli (p < 0.05). Reduced theta oscillations during auditory processing provide strong quantitative evidence of activation deficits in related networks in bipolar disorder. Fast theta responses are related to cognitive functions, whereas slow theta responses are related to sensory processes more than cognitive processes.
Keywords: Bipolar disorder, Event related oscillations, Oddball, Theta, Theta oscillations, Medication-free, Euthymia, Cognitive dysfunction
Introduction
Cognitive deficits and emotional dysregulation in euthymia are indicators of enduring pathology in bipolar disorder (BD). Disruptions of the connections between frontal cortex, amygdala, basal ganglia, thalamus, entorhinal cortex and hippocampus are suggested to participate in the underlying pathology of bipolar disorder (Dupont et al. 1995; Caligiuri et al. 2004; Blumberg et al. 2002; Phillips et al. 2003; Strakowski et al. 2005). These connections are also believed to serve in modulation of cognition and emotional consonance (Strakowski et al. 2005).
In contrast to the wealth of neuroimaging studies in BD, little is known about electrophysiologic correlates. Cognitive deficits in BD may indicate that EEG recorded under cognitive task conditions would be better suited to identify electrophysiological correlates of cognitive dysfunctions. Event-related potential studies of P300 peak amplitudes produced inconsistent results in BD; Some studies reported reduced P300 amplitudes (Muir et al. 1991; El-Badri et al. 2001; Salisbury et al. 1998, 1999; O’Donnell et al. 2003, 2004b; Fridberg et al. 2009), while some others reported no difference between healthy controls and patients with BD (Souza et al. 1995; Strik et al. 1998; Hall et al. 2007; Kaya et al. 2007; Schulze et al. 2007, 2008).
Dysfunction in sensory or cognitive processes cannot be explained only by a frequency response; however, connectivity deficits between involved brain sites may be reflected in a frequency response (Başar 2006). Over the last decade, oscillatory activity has increasingly been applied in various clinical pathologies, including bipolar disorder (see reviews Başar and Güntekin 2008; Başar 2010). The degree of resting state long-range synchrony was found to be significantly reduced in manic patients in comparison to healthy controls at all frequencies (Bhattacharya 2001). Medicated euthymic patients had increased delta and decreased beta synchronization in the frontal sites (Chen et al. 2009). Patients in manic or mixed state were found to have deficits in auditory EEG synchronization in beta and gamma range activity during click entrainment paradigm (O’Donnell et al. 2004a). Gamma band power reduction has also been found in euthymia (Lee et al. 2010). Auditory steady state response (ASSR) is thought to be generated by neural networks, including auditory cortices and thalamo-cortical circuits (Pantev et al. 1996; Rass et al. 2010). Deficits in generation and maintenance of ASSR in bipolar disorder may indicate disturbances in neural networks involved in auditory cortices (O’Donnell et al. 2004a; Rass et al. 2010). When comparing evoked neural oscillations in the left hemisphere in response to speech sounds, patients with BD displayed larger evoked oscillations than both schizophrenics and healthy controls in an MEG study (Oribe et al. 2010). In almost all of these studies, patients were medicated. Yener et al. (2007) showed that theta oscillations were significantly greater in patients with mild AD on cholinomimetic medication compared with those of medication-free patients with AD.
Previous studies by our group investigating oscillatory responses to visual oddball stimuli in medication-free bipolar patients found increased occipital beta activity in manic (Özerdem et al. 2008) and reduced long distance gamma coherence in manic (Özerdem et al. 2010) and euthymic states (Özerdem et al. 2011). Başar et al. (2012) recently showed that, in spontaneous EEG, bipolar patients had significantly reduced alpha activity in comparison to healthy controls.
Theta oscillations have been proposed to provide integration and communication between different brain areas (Başar 2010; Başar-Eroğlu and Demiralp 2001; Başar et al. 2001; Kirk and Mackay 2003; Sarnthein et al. 1998). Theta rhythm has been considered to be the fingerprint of all limbic structures; it is most prominent in the hippocampal formation (Lopes da Silva 1990). Theta oscillations are related to memory, attention and cognitive control processes (e.g., see Başar et al. 2001; Başar 1998, 1999; Klimesch 1999; Kahana et al. 1999), thus they are of particular interest in cognitive paradigms. Numerous structures in frontal (e.g., Gevins et al. 1997; Onton et al. 2005; Mitchell et al. 2008) and medial temporal regions (e.g., Basar-Eroglu et al. 1992; Kahana et al. 1999; Raghavachari et al. 2001; von Stein and Sarnthein 2000) generate cognition-related theta oscillations. Theta activity reflects functional integration of the abovementioned structures into coherent neurocognitive networks (see e.g. Başar et al. 2001; Başar 1998; Klimesch 1999; von Stein and Sarnthein 2000 for reviews). Thus, altered theta responses are likely to represent neurophysiologic correlates of cognitive deficits in BD (Sakowitz et al. 2000).
To our knowledge, no studies to date have compared the theta band power of control and BD samples. Electrophysiological assessments of oscillations provide high temporal resolution and therefore assessments of oscillatory responses to sensory or cognitive events constitute a useful imaging modality. Assessment of brain responses in the absence of any potential symptom or medication-related confounding effects may provide a major advantage to understand the underlying pathophysiology of bipolar disorder. The aim of this study was to assess evoked and event-related oscillatory responses to auditory stimuli in medication-free euthymic bipolar patients in comparison to healthy controls. Since verbal learning and verbal memory deficits are the most consistent cognitive dysfunctions in BD (Robinson et al. 2006; Bora et al. 2009), it can be hypothesized that theta responses to auditory oddball paradigm within the patient group may differ from healthy controls.
Method
Subjects
Twenty-two euthymic, drug-free patients with euthymic bipolar I (n = 19) or bipolar II (n = 3) diagnoses (female/male = 16/6; mean age ± SD: 31.18 ± 6.34, range: 23–44 years), and sex (female/male: 16/6), age (mean age of healthy controls: 29.41 ± 7.77, range = 20–45) and education (mean years of education for bipolar patients = 12.7 ± 3.9 vs. healthy controls = 14.1 ± 1.7 years)—matched healthy controls were enrolled into the study (Table 1). All subjects were interviewed with the Turkish version of the SCID-I (Structured Interview according to DSM-IV) (First et al. 1996). The local Ethical Committee of Bakırkoy Research and Training Hospital approved the study. Each participant provided written informed consent. Patients needed to be euthymic at least for 6 months, psychotropic-free for at least 2 weeks prior to study enrollment; to score 7 or less on the reliable and validated Turkish versions of the Young Mania Rating Scale (YMRS) (Young et al. 1978; Karadağ et al. 2002), Hamilton Depression Rating Scale (HAM-D 21) (Hamilton 1960; Aydemir and Deveci 2003); to have no co-morbid axis I diagnosis, and to be medically healthy, as confirmed by physical examination and routine laboratory tests. Exclusion criteria were: Pregnancy, lactation, consumption of alcohol or illicit substances within the previous 2 weeks, history of alcohol- or substance misuse, axis 1 psychiatric comorbidity and neurological conditions such as neurodegenerative diseases, epilepsy, and brain surgery. Volunteers who proved to have no present or past psychiatric condition on SCID-I interview and to be medically healthy on physical examination were enrolled as the control group.
Table 1.
Subjects’ characteristics
| Patients with bipolar disorder | Healthy controls | p | |
|---|---|---|---|
| Agea | 31.18 ± 6.34 | 29.41 ± 7.77 | 0.412 |
| Educationa | 12.73 ± 3.68 | 14.55 ± 2.13 | 0.126 |
| Age at disease onseta | 21.77 ± 6.28 | ||
| Duration of euthymiab | 44.95 ± 37.39 | ||
| Duration of illnessb | 117.95 ± 57.18 | ||
| Number of | |||
| Total episodes | 4.05 ± 3.12 | ||
| Manic episodes | 2.27 ± 1.96 | ||
| Depressive episodes | 1.09 ± 1.11 | ||
| Hypomanic episodes | 0.68 ± 1.17 | ||
Mean ± SD
aYears
bMonths
Stimuli and paradigms
Participants were seated in a dimly-lit isolated room with eyes open. Two types of stimuli were presented: simple auditory stimuli for analyzing auditory evoked potentials (AEP), and auditory oddball paradigm for analyzing auditory event-related potentials (AERP). The auditory stimuli had 16 ms rising time, 50 ms falling time and 1,000 ms duration, and were presented by two loudspeakers.
The auditory simple stimuli were tones of 80 dB and 1,500 Hz. The inter-stimulus intervals varied randomly between 3 and 7 s. The total number of stimuli was 60.
The classical auditory oddball paradigm that was used in the experiments consisted of two types of stimuli: task-relevant target and task- irrelevant non-target (standard). The total number of stimuli was 120 (40 target, 80 non-target). Target (80 dB, 1,600-Hz tones) and non-target (1,500-Hz tones) were presented in a random sequence. The interval between tones varied randomly between 3 and 7 s. The subjects were instructed to keep a mental count of the number of target (1,600 Hz) tones.
The evoked and event-related theta responses to the target, non-target and simple auditory stimulation stimuli were analyzed and compared.
Electrophysiological recording
EEG was recorded with 30 Ag–AgCl electrodes mounted in an elastic cap (Easy-cap) according to the international 10–20 system. Additionally, two linked earlobe electrodes (A1 + A2) served as references. The EOG from the medial upper and lateral orbital rim of the right eye was also registered. For the reference electrodes and EOG recordings, Ag–AgCl electrodes were used. All electrode impedances were <10 kΩ. The EEG was amplified by means of a BrainAmp 32-channel DC device with band limits of 0.01–250 Hz. The EEG was digitized on-line at a sampling rate of 500 Hz.
Evoked and event-related oscillatory analysis by means of adaptive filtering
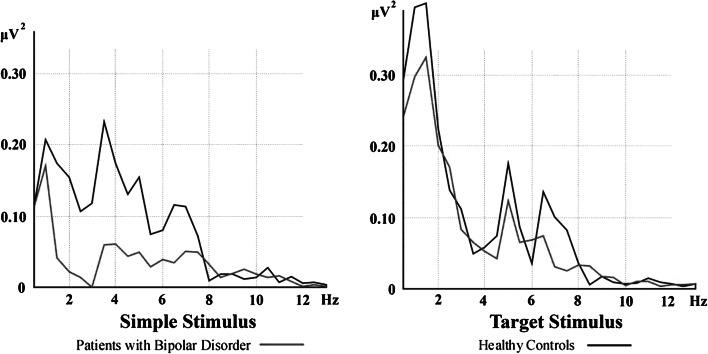
Artifacts were eliminated by manual off-line selective averaging, taking into consideration the EOG recorded from the right eye. The sweep numbers were equalized randomly between the target, non-target and simple auditory stimulation conditions. The epochs (between 0 and 800 ms) of each subject were averaged and then the digital FFT-based power spectrum analysis was performed. (10 % Hanning windowing function was evaluated in order to calculate the theta frequency peak). Subject averages and grand averages were calculated for each electrode site and experimental condition. As seen in Fig. 1, in the grand average of response power spectrum upon stimulation of target stimuli, two different peaks were detected in theta frequency in the healthy control group, both for slow theta (4–6 Hz) and fast theta (6–8 Hz). Adaptive filtering was applied in analyzing the data in both healthy and patient groups, due to the two different peaks observed in theta frequency range only in healthy controls. Adaptive filtering of the response provides a major advantage that subsystems of the system might be selectively removed to obtain isolation. Separate isolation of the filters may allow the choice of amplitude and frequency characteristics of the filters. Ideal filters may be applied without phase shifts. Furthermore, this method also permits the definition of filters with exact characteristics their adequate regulation according to the amplitude characteristics of the system (for further information see Başar 2004).
Fig. 1.
Power spectrum of auditory evoked and event-related responses over left frontal (F3) location
Accordingly, each subject’s averaged evoked and event-related potentials were digitally filtered in slow theta (4–6 Hz) and fast theta (6–8 Hz) frequency ranges. The maximum peak-to-peak amplitudes for each subject’s averaged slow theta (4–6 Hz) and fast theta (6–8 Hz) responses were analyzed; that is, the largest peak-to-peak value in these frequency ranges in terms of μVs found in the time window between 0 and 500 ms.
Statistical analysis
SPSS was used for statistical analysis. A repeated measure ANOVA was used to determine the statistical significance of differential theta responses over different conditions, locations, and between patients and controls. Two separate ANOVAs were used for the two different frequency theta ranges (4–6 Hz and 6–8 Hz). In the analysis of theta responses, repeated measures of ANOVA included the healthy controls and euthymic patients as the between-subjects factor; stimulus types (target, non-target, simple auditory stimulation) at three levels, locations [frontal (F3–F4), central (C3–C4), temporal (T7–T8), temporo-parietal (TP7–TP8), Parietal (P3–P4), Occipital (O1–O2)] signals at six levels and hemispheres (right, left) at two levels were included as within-subject factors. Greenhouse–Geisser corrected p-values are reported. The t test was used for post hoc comparisons. In all analyses, the level of significance was p < 0.05 with 95 % confidence interval. In the analysis of behavioral data, due to extreme values, logarithmic transformation was applied to numbers of errors and Spearman’s correlation analysis was used for correlations. Spearman’s correlation test was used to obtain correlations between the clinical data and evoked and event-related theta oscillatory responses. Each subjects’ frontal, central, temporal, temporo-parietal, parietal and occipital values were obtained by calculating average values of left and right electrode values and these averages were used to obtain correlations between clinical variables.
Results
Clinical characteristics of the patients are given in Table 1. All patients were drug-free for at least 2 weeks and euthymic for at least 6 months; mean score for the 21-item HAM-D was 2.55 (±2.3) and mean score for YMRS was 0.55 (±1.19).
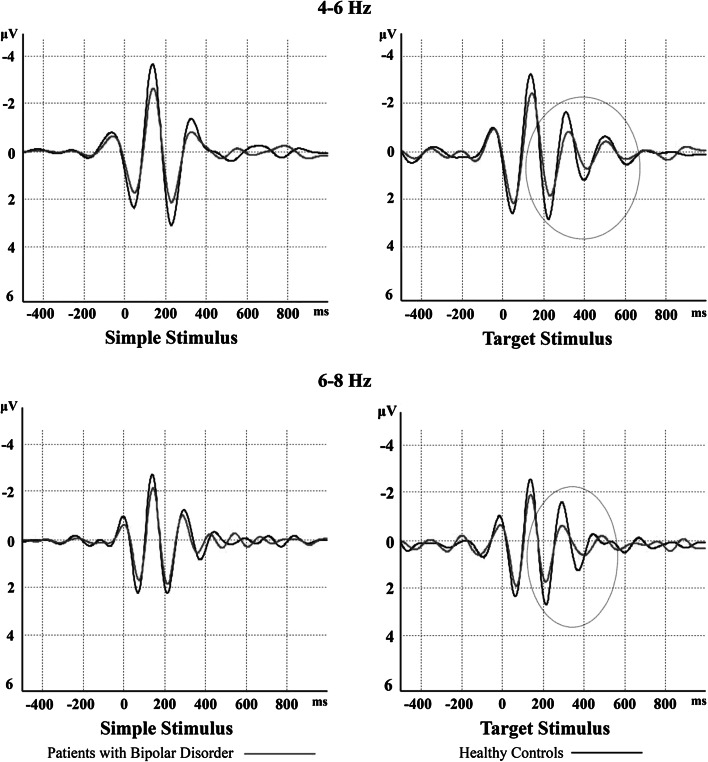
Figure 2 shows a sample of filtered and averaged theta response at the left-frontal location. There was a 20 % decrease in evoked oscillations and a 30 % decrease in event-related oscillations in patients with bipolar disorder in comparison to healthy controls.
Fig. 2.
Filtered theta response in left-frontal (F3) electrode site upon simple and target stimuli. Grand averages of theta responses of patients with bipolar disorder are represented by red lines and those of healthy controls are represented by black lines. There is a 20 % decrease in evoked oscillations and 30 % decreases in event-related oscillations in patients with bipolar disorder compared to healthy controls
Slow theta oscillations (4–6 Hz)
General features
In the repeated measures of ANOVA, there was a significant stimulus-type effect [F(2,84): 17.672; p < 0.0001] in the whole group (n = 44). Post-hoc comparisons showed that slow theta responses upon target stimuli were significantly higher than slow theta responses upon non-target stimuli in the whole group (p < 0.0001). Furthermore, slow theta responses upon simple auditory stimuli were significantly higher than slow theta responses upon non-target stimuli (p < 0.0001). No difference was detected between simple and target stimuli. The location effect was also significant [F(5,210): 126.738; p < 0.0001] in the whole group (n = 44). Post-hoc comparisons showed that, regardless of the stimulus type, slow theta responses at frontal and central electrodes were higher than temporal, temporoparietal, parietal and occipital electrodes (p < 0.0001 for all comparisons). Furthermore, slow theta responses at temporal and temporoparietal electrodes were higher than those at parietal and occipital electrodes (p < 0.0001 for all comparisons). There was a significant [location × stimulus-type] effect (F(10,420): 7.352; p < 0.0001) in the whole group (n = 44). Post-hoc comparisons showed that fast theta responses to target stimuli were significantly higher than fast theta responses to auditory non-target stimuli at frontal, central and occipital electrode sites (p < 0.002; p < 0.002; p < 0.0001). It is also note that in all electrodes slow theta responses of non-target stimuli were lower than the slow theta responses of target and simple auditory stimuli.
Comparison of the patient and healthy control groups
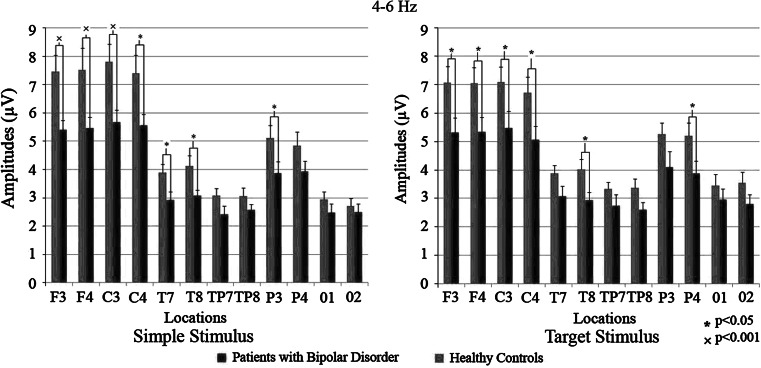
Slow theta response differed significantly between the patient and control groups (F(1,42): 5.686; p < 0.05). The t test showed that patients had significantly lower slow theta activity in response to simple stimuli (EP) at F3 (p < 0.01), F4 (p < 0.01), C3 (p < 0.01), C4 (p < 0.05), T7 (p < 0.05), T8 (p < 0.05) and P3 (p < 0.05) electrodes. Also for the target stimulus, the patients had significantly lower values at the same locations [F3 (p < 0.05), F4 (p < 0.05), C3 (p < 0.05), C4 (p < 0.05)]. For the non-target stimuli, the difference was significant at T7 (p < 0.05) and T8 (p < 0.05) locations. Differences between groups are represented in Fig. 3.
Fig. 3.
Mean amplitudes of patients with bipolar disorder and healthy controls in 4–6 Hz frequency range. Red bars represent patients with bipolar disorder and blue bars represent healthy controls. “*” sign represent p values < 0.05; “×” sign represent p values < 0.001
Fast theta oscillations (6–8 Hz)
General features
ANOVA showed that fast theta responses differed significantly between stimulus-type effects [F(2,84): 9.691; p < 0.0001] in the whole group (n = 44). Post-hoc comparisons showed that fast theta responses upon target stimuli were significantly higher than fast theta responses upon non-target stimuli (p < 0.0001). Furthermore, fast theta responses upon simple auditory stimuli were significantly higher than fast theta responses upon non-target stimuli (p < 0.0001). No significant difference was detected between simple and target stimuli. The location effect was also significant [F(5,210): 93.298; p < 0.0001] in the whole group (n = 44). Post-hoc comparisons showed that fast theta responses at frontal and central electrodes were higher than at temporal, temporoparietal, parietal and occipital electrodes (p < 0.0001 for all electrodes). Furthermore, fast theta responses at temporal and temporoparietal electrodes were higher than those at parietal and occipital electrodes (p < 0.001; for all comparisons). (Frontal = Central > temporal > temporoparietal > Parietal > Occipital) There was a significant [location × stimulus-type] effect [F(10,420): 2.882; p < 0.05] in the whole group (n = 44). Post-hoc comparisons showed that fast theta responses to target stimuli were significantly higher than fast theta responses to auditory non-target stimuli at frontal, central and occipital electrode sites (p < 0.003; p < 0.007; p < 0.0001). It is also note that in all electrodes fast theta responses of non-target stimuli were lower than the fast theta responses of target and simple auditory stimuli.
Comparison of the patient and healthy control groups
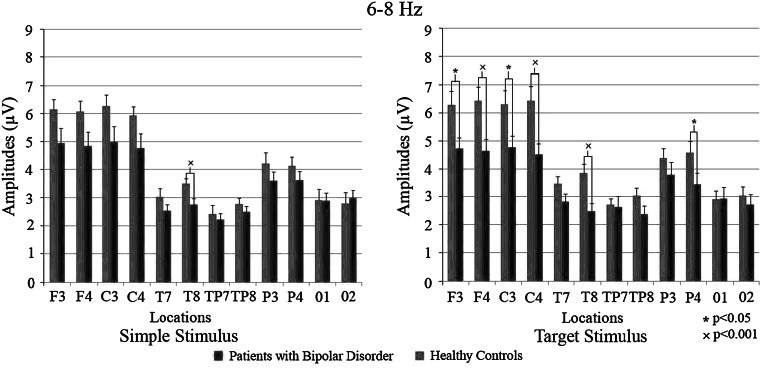
The ANOVA of fast theta responses revealed significant [stimulus × location × group] effect [F(10.420): 2.867; p < 0.05). Post-hoc comparisons showed that, upon simple stimuli, fast theta responses of healthy controls were greater than patients with BD only in right temporal region (p < 0.01 for all sites); upon target stimuli, fast theta responses of healthy controls were greater than patients with BD at frontal, central, right temporal and right parietal regions (p < 0.001, p < 0.001, p < 0.01 and p < 0.05 respectively). Upon non-target stimuli, healthy controls showed greater fast theta responses than patients with BD only in the temporal region (p < 0.05). Comparison of [stimuli × location × hemisphere] revealed that the responses of the healthy control group were significantly higher than the patient group [F(10.420): 2.093; p < 0.05]. T test results showed that patients had significantly lower theta activity in response to simple stimuli (EP) only at site T8 (p < 0.01). In response to the target stimuli, the patients had significantly lower values at locations F3 (p < 0.05), F4 (p < 0.01), C3 (p < 0.05), C4 (p < 0.01), T8 (p < 0.01) and P4 (p < 0.05). No significant difference was found between the groups in response to the non-target stimuli. Inter-group differences are represented in Fig. 4.
Fig. 4.
Mean amplitudes of patients with bipolar disorder and healthy controls in 6–8 Hz frequency range. Red bars represent patients with bipolar disorder and blue bars represent healthy controls. “*” sign represent p values <0.05; “×” sign represent p values <0.001
Behavioral data
During the elicitation period of event-related oscillations, subjects were instructed to count the target stimuli as a cognitive task. It was found that 18 of 22 members of the patient group (mean = 3.82 ± 7.39, range: 1–26) made errors, compared with 11 of the 22 control subjects (mean = 6.56 ± 9.54, range: 1–40).
Correlation analyses
Each subjects’ frontal, central, temporal, temporo-parietal, parietal and occipital electrodes were used to obtain correlations between clinical variables such as age, education, age at disease onset, duration of euthymia, duration of the disease, total numbers of episodes, total numbers of manic and depressive episodes. Slow theta and fast theta responses to simple, target and non-target stimuli correlated significantly with age and age at disease onset (Table 2).
Table 2.
Correlations between mean amplitudes of averaged filtered theta response and clinical variables
| Frontal | Central | Temporal | Temporo-parietal | Parietal | Occipital | |
|---|---|---|---|---|---|---|
| Simple stimulus | ||||||
| 4–6 Hz | ||||||
| Age | −0.49* | −0.70¥ | −0.50* | −0.60¥ | −0.55¥ | −0.43* |
| Age at disease onset | −0.40 | −0.52* | −0.13 | −0.36 | −0.55¥ | −0.49* |
| 6–8 Hz | ||||||
| Age | −0.33 | −0.58¥ | −0.40 | −0.42 | −0.40 | −0.42 |
| Age at disease onset | −0.28 | −0.38 | −0.05 | −0.17 | −0.43* | −0.33 |
| Target stimulus | ||||||
| 4–6 Hz | ||||||
| Age | −0.53* | −0.53* | −0.51* | −0.39 | −0.53* | −0.35 |
| Age at disease onset | −0.26 | −0.30 | −0.21 | −0.22 | −0.40 | −0.13 |
| 6–8 Hz | ||||||
| Age | −0.23 | −0.50* | −0.42* | −0.50* | −0.43* | −0.38 |
| Age at disease onset | −0.17 | −0.47* | −0.23 | −0.30 | −0.47* | −0.25 |
| Non-target stimulus | ||||||
| 4–6 Hz | ||||||
| Age | −0.36 | −0.48* | −0.48* | −0.57¥ | −0.54¥ | −0.50* |
| Age at disease onset | −0.43* | −0.52* | −0.18 | −0.44* | −0.61¥ | −0.29 |
| 6–8 Hz | ||||||
| Age | −0.16 | −0.28 | −0.35 | −0.48* | −0.43* | −0.41 |
| Age at disease onset | −0.16 | −0.29 | −0.17 | −0.25 | −0.38 | −0.22 |
Significant correlations are marked with bold characters
Spearman’s correlation test; r values. *p < 0.05; ¥p < 0.001. Education, age at disease onset, duration of euthymia, total numbers of episodes, total numbers of manic and depressive episodes did not show any correlation
Discussion
The major finding of the present study is that patients with bipolar disorder showed significantly lower theta oscillatory responses upon auditory stimulation and in response to target and simple stimuli during an oddball paradigm in comparison to healthy controls. Patients presented different slow (4–6 Hz) and fast (6–8 Hz) theta response patterns. As the slow theta (4–6 Hz) responses of the patients were significantly lower upon simple auditory stimulation and target stimuli during the oddball paradigm at bilateral frontal, central, right temporal and right parietal regions, the 6–8 Hz activity of the patients showed significant reductions in the same locations compared to healthy controls only upon target stimulus.
A comparison of Figs. 3 and 4 clearly imposes the following reasoning: in the slow theta frequency window (4–6 Hz), all cortical areas react with significant increase, which is independent of the modality of stimulation. Simple stimulation and target stimulation both show significant increases of as much as 50 %. In contrast, within the fast theta frequency band (6–8 Hz), all locations showed significant responses upon target stimulus; however, for except T8, none of the cortical areas are increased upon simple stimulation. These results clearly indicate that both theta responses are involved with different functional processing.
Theta frequency bands have been extensively studied and are believed to be involved in cognitive functions such as working memory (Başar-Eroğlu and Demiralp 2001; Başar et al. 2001; Başar 1998, 1999; Klimesch 1999; Kahana et al. 1999; Klimesch et al. 1996, 1997, 2001a, b). According to the model of Klimesch et al. (1996), short-term memory demands lead to synchronization in the theta band, manifested as an increase in band power, and occurs at the anterior limbic system. Oscillations in the theta and alpha band may provide the basis for encoding, accessing and retrieving cortical codes that are stored in the form of widely distributed but intensively interconnected cell assemblies (Başar 1999). In a recent study, Caravaglios et al. (2010) compared theta frequency responses (to oddball paradigm) of patients with Alzheimer disease and healthy controls by means of oddball paradigm. They found that although responses of healthy controls were responsive to target stimuli, theta frequency activity of the patients was not responsive to target or non-target stimuli. Patients showed increased pre-stimulus theta frequency activity, and no enhancement was detected in the early (0–250 ms) or late (250–500 ms) post-stimulus interval. The authors commented that patients had insufficient resources for adequate attention. It was also indicated that, unlike the healthy controls, patients did not have prominent frontal lobe activity during stimulus processing. Theta and delta are the most affected frequencies upon oddball paradigm in Alzheimer disease and mild cognitive impairment as instances of cognitive dysfunctions (Başar et al. 2010). The authors suggested that cognitive impairment in Alzheimer disease was particularly manifested by reduced coherences in delta and theta frequency ranges.
Event-related oscillations in the theta band are prolonged and/or have a second time-window approximately 300 ms after the presentation of the target stimulus in oddball experiments (Başar-Eroğlu and Demiralp 2001; Başar et al. 2001). Prolongation of theta response is interpreted as the reflection of the correlation with selective attention. Başar-Eroğlu and Demiralp (2001) further showed that the second theta window is more associated with target stimuli. In addition, mental count of the target stimuli is associated with sustained attention and working memory. Patients with bipolar disorder are known to suffer from cognitive dysfunctions, particularly in sustained attention, executive functions, working memory, verbal learning and verbal memory sub-domains of cognitive functions (see meta-analyses, Robinson et al. 2006; Bora et al. 2009).
In general, theta frequency responses upon visual stimuli increase diffusely, including frontal, parietal, occipital and vertex sites; in contrast, auditory stimulus increases theta responses at frontal and parietal sites (Başar 1999; Demiralp and Başar 1992). The CA3 layers of the hippocampus, frontal- and parietal lobes are interconnected. The CA3 layer of hippocampus is shown to produce theta oscillations upon cognitive functions such as attention, perception, learning and memory in intracranial recordings from the cat brain (Başar-Eroğlu and Başar 1991). Distribution of theta responses at frontal and parietal lobes upon auditory stimulus may also be related to the hippocampus (Başar 1999). Therefore, these findings are strong quantitative indicators of dysfunctional cognitive processes occurring in cortico-subcortical loops in bipolar disorder. Basar-Eroglu et al. (2008) showed that distribution of theta and alpha responses upon a visual paradigm had a different pattern from healthy controls in schizophrenia. Healthy controls’ responses upon simple and non-target stimuli were distributed over the occipital lobe, while patients’ responses were distributed over fronto-central sites. The authors’ comment this on altered topography of the late theta response was that, even with simple task, patients required high cognitive effort to process stimuli.
Functional neuroimaging studies showed association between discrimination of auditory stimulation and frontoparietal activation, particularly over the right hemisphere (Paquette et al. 1996; Boucher and Bryden 1997; Zatorre 2001). In 6–8 Hz responses, the difference between groups became more prominent in response to target stimuli on the right hemisphere, which is a marker of activation deficit among patients with bipolar disorder when perceiving and discriminating pitch. Hence, the decreased theta responses of the patients indicate disruptions in the frontoparietal networks. In addition, these results suggest that, rather than 4–6 Hz, the 6–8 Hz band is more specific to the cognitive components of the oddball paradigm.
According to Yener and Başar (2010), sensory event may evoke brain areas reacting sensory inputs, whereas cognitive processes may evoke areas to respond to both sensory and cognitive inputs. According to this assumption, 4–6 Hz may include both sensory and cognitive components, whereas 6–8 Hz may include only cognitive components. In our study, the 4–6 Hz responses of the patient group were significantly decreased upon both simple and target stimuli; however, 6–8 Hz responses differed only upon target stimulus. Therefore, 4–6 Hz may be related to sensory events (Aftanas et al. 2001, Aftanas and Golocheikine 2001, Aftanas et al. 2003a, b), while 6–8 Hz may be related to cognitive processing (Aftanas et al. 2003b). Previous studies found that 4–6 Hz is more prominent in posterior sites, while 6–8 Hz is greater in frontal sites (Kamarajan et al. 2008; Krause et al. 2000). Loops integrating hippocampus and prefrontal cortices may serve cognitive functions via 6–8 Hz activity.
Several previous studies by our group reported that patients with bipolar disorder showed activation and synchronization deficits in different frequency ranges including delta, alpha, beta and gamma bands (Özerdem et al. 2008, 2010, 2011; Başar et al. 2012). This is the first study in the theta frequency range. A major strength of this study is the inclusion of medication-free patients, whereas the relatively small sample size is a limitation.
Concluding remarks
The results of this study represent a specific feature for BD: Auditory processing deficiency may indicate disruption of synchronization during auditory cognitive activity. These findings provide neurophysiological evidence of auditory processing dysfunction in BD. On the other hand, the oscillatory brain dynamics of patients with BD differ from healthy controls in both auditory and visual paradigms.
The results suggest that fast theta (6–8 Hz) frequency responses are associated with cognitive functions, and that slow theta (4–6 Hz) frequency responses are more closely associated with sensory functions than with cognitive functions. Theta frequency should be analyzed in two different bands, including 4–6 and 6–8 Hz bands.
References
- Aftanas LI, Golocheikine SA. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neurosci Lett. 2001;310:57–60. doi: 10.1016/S0304-3940(01)02094-8. [DOI] [PubMed] [Google Scholar]
- Aftanas L, Varlamov A, Pavlov S, Makhnev V, Reva N. Event-related synchronization and desynchronization during affective processing: emergence of valence-related time-dependent hemispheric asymmetries in theta and upper alpha band. Int J Neurosci. 2001;110:197–219. doi: 10.3109/00207450108986547. [DOI] [PubMed] [Google Scholar]
- Aftanas LI, Pavlov SV, Reva NV, Varlamov AA. Trait anxiety impact on the EEG theta band power changes during appraisal of threatening and pleasant visual stimuli. Int J Psychophysiol. 2003;50:205–212. doi: 10.1016/S0167-8760(03)00156-9. [DOI] [PubMed] [Google Scholar]
- Aftanas LI, Varlamov AA, Reva NV, Pavlov SV. Disruption of early event-related theta synchronization of human EEG in alexithymics viewing affective pictures. Neurosci Lett. 2003;340:57–60. doi: 10.1016/S0304-3940(03)00070-3. [DOI] [PubMed] [Google Scholar]
- Aydemir Ö, Deveci A (2003) Validity and reliability of structured interview for Hamilton depression rating scale seasonal affective disorders version. VIIth annual spring symposium of psychiatric association of Türkiye abstract book, p 187
- Başar E. Brain oscillations I: principles and approaches. Heidelberg: Springer; 1998. [Google Scholar]
- Başar E. Brain function and oscillations: II. Integrative brain function. Neurophysiology and cognitive processes. Heidelberg: Springer; 1999. [Google Scholar]
- Başar E. Memory and brain dynamics: oscillations integrating attention, perception, learning and memory. Florida: CRC Press; 2004. [Google Scholar]
- Başar E. The theory of whole-brain-work. Int J Psychophysiol. 2006;60:133–138. doi: 10.1016/j.ijpsycho.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Başar E. Why the concept of “Quantum Brain” was not discovered in 1940 s. NeuroQuantology. 2010;8:322–336. [Google Scholar]
- Başar E, Güntekin B. A review of brain oscillations in cognitive disorders and the role of neurotransmitters. Brain Res. 2008;1235:172–193. doi: 10.1016/j.brainres.2008.06.103. [DOI] [PubMed] [Google Scholar]
- Başar E, Schürmann M, Sakowitz O. The selectively distributed theta system: functions. Int J Psychophysiol. 2001;39:197–212. doi: 10.1016/S0167-8760(00)00141-0. [DOI] [PubMed] [Google Scholar]
- Başar E, Güntekin B, Tülay E, Yener GG. Evoked and event-related coherence of alzheimer patients manifest differentiation of sensory-cognitive networks. Brain Res. 2010;1357:79–90. doi: 10.1016/j.brainres.2010.08.054. [DOI] [PubMed] [Google Scholar]
- Başar E, Güntekin B, Atagün İ, Turp Gölbaşı B, Tülay E, Özerdem A. Brain’s alpha activity is highly reduced in euthymic bipolar disorder patients. Cogn Neurodyn. 2012;6:11–20. doi: 10.1007/s11571-011-9172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Başar-Eroğlu C, Başar E. A compound P300–40 Hz response of the cat hippocampus. Int J Neurosci. 1991;60:227–237. doi: 10.3109/00207459109167035. [DOI] [PubMed] [Google Scholar]
- Başar-Eroğlu C, Demiralp T. Event-related theta oscillations: an integrative and comparative approach in the human and animal Brain. Int J Psychophysiol. 2001;39:167–195. doi: 10.1016/S0167-8760(00)00140-9. [DOI] [PubMed] [Google Scholar]
- Başar-Eroğlu C, Başar E, Demiralp T, Schürmann M. P300-response: possible psychophysiological correlates in delta and theta frequency channels. A review. Int J Psychophysiol. 1992;13:161–179. doi: 10.1016/0167-8760(92)90055-G. [DOI] [PubMed] [Google Scholar]
- Başar-Eroğlu C, Schmiedt-Fehr C, Marbach S, Brand A, Mathes B. Altered oscillatory alpha and theta networks in schizophrenia. Brain Res. 2008;1235:143–152. doi: 10.1016/j.brainres.2008.06.114. [DOI] [PubMed] [Google Scholar]
- Bhattacharya J. Reduced degree of long-range phase synchrony in pathological human brain. Acta Neurobiol Exp (Wars) 2001;61:309–318. doi: 10.55782/ane-2001-1406. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Charney DS, Krystal JH. Frontotemporal neural systems in bipolar disorder. Semin Clin Neuropsychiatry. 2002;7:243–254. doi: 10.1053/scnp.2002.35220. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Boucher R, Bryden MP. Laterality effects in the processing of melody and timbre. Neuropsychologia. 1997;35:1467–1473. doi: 10.1016/S0028-3932(97)00066-3. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Brown GG, Meloy MJ, Eyler LT, Kindermann SS, Eberson S, Frank LR, Lohr JB. A functional magnetic resonance imaging study of cortical asymmetry in bipolar disorder. Bipolar Disord. 2004;6:183–196. doi: 10.1111/j.1399-5618.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- Caravaglios G, Castro G, Costanzo E, Di Maria G, Mancuso D, Muscoso EG. θ power responses in mild Alzheimer’s disease during an auditory oddball paradigm: lack of theta enhancement during stimulus processing. J Neural Transm. 2010;117:1195–1208. doi: 10.1007/s00702-010-0488-2. [DOI] [PubMed] [Google Scholar]
- Chen AC, Tang Y, Rangaswamy M, Wang JC, Almasy L, Foroud T, Edenberg HJ, Hesselbrock V, Nurnberger J, Jr, Kuperman S, O’Connor SJ, Schuckit MA, Bauer LO, Tischfield J, Rice JP, Bierut L, Goate A, Porjesz B. Association of single nucleotide polymorphisms in a glutamate receptor gene (GRM8) with theta power of event-related oscillations and alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150:359–368. doi: 10.1002/ajmg.b.30818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demiralp T, Başar E. Theta rhythmicities following expected visual and auditory targets. Int J Psychophysiol. 1992;13:147–160. doi: 10.1016/0167-8760(92)90054-F. [DOI] [PubMed] [Google Scholar]
- Dupont RM, Jernigan TL, Heindel W, Butters N, Shafer K, Wilson T, Hesselink J, Gillin JC. Magnetic resonance imaging and mood disorders. Localization of white matter and other subcortical abnormalities. Arch Gen Psychiatry. 1995;52:747–755. doi: 10.1001/archpsyc.1995.03950210041009. [DOI] [PubMed] [Google Scholar]
- El-Badri SM, Ashton CH, Moore PB, Mursh VR, Ferrier IN. Electrophysiological and cognitive function in young euthymic patients with bipolar affective disorder. Bipolar Disord. 2001;3:79–87. doi: 10.1034/j.1399-5618.2001.030206.x. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Gibbon M, Williams JBW. User’s guide for the structured interview for DSM-IV axis I disorders—research version (SCID-I, version 2.0, February 1996 final version) New York: Biometrics Research; 1996. [Google Scholar]
- Fridberg DJ, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, Malloy FW, O’Donnell BF. Relationships between auditory event-related potentials and mood state, medication, and comorbid psychiatric illness in patients with bipolar disorder. Bipolar Disord. 2009;11:857–866. doi: 10.1111/j.1399-5618.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Hall MH, Rijsdijk F, Picchioni M, Schulze K, Ettinger U, Toulopoulou T, Bramon E, Murray RM, Sham P. Substantial shared genetic influences on schizophrenia and event-related potentials. Am J Psychiatry. 2007;164:804–812. doi: 10.1176/appi.ajp.164.5.804. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature. 1999;399:781–784. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Rangaswamy M, Chorlian DB, Manz N, Tang Y, Pandey AK, Roopesh BN, Stimus AT, Porjesz B. Theta oscillations during the processing of monetary loss and gain: a perspective on gender and impulsivity. Brain Res. 2008;1235:45–62. doi: 10.1016/j.brainres.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadağ F, Oral ET, Yalçın FA, Erten E. Validity, Reliability of young mania rating scale in Turkey. Turk Psikiyatri Derg. 2002;13:107–114. [PubMed] [Google Scholar]
- Kaya E, Aydemir O, Selcuki D. Residual symptoms in bipolar disorder: the effect of the last episode after remission. Biol Psychiatry. 2007;31:1387–1392. doi: 10.1016/j.pnpbp.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Kirk IJ, Mackay JC. The role of theta-range oscillations in synchronising and integrating activity in distributed mnemonic networks. Cortex. 2003;39:993–1008. doi: 10.1016/S0010-9452(08)70874-8. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29:169–195. doi: 10.1016/S0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T. Theta band power in the human scalp EEG and the encoding of new information. NeuroReport. 1996;7:1235–1240. doi: 10.1097/00001756-199605170-00002. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Ripper B. Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci Lett. 1997;238:9–12. doi: 10.1016/S0304-3940(97)00771-4. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Stadler W, Pöllhuber D, Sauseng P, Röhm D. Episodic retrieval is reflected by a process specific increase in human electroencephalographic theta activity. Neurosci Lett. 2001;302:49–52. doi: 10.1016/S0304-3940(01)01656-1. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Yonelinas A, Kroll NE, Lazzara M, Röhm D, Gruber W. Theta synchronization during episodic retrieval: neural correlates of conscious awareness. Brain Res Cogn Brain Res. 2001;12:33–38. doi: 10.1016/S0926-6410(01)00024-6. [DOI] [PubMed] [Google Scholar]
- Krause CM, Sillanmäki L, Koivisto M, Saarela C, Häggqvist A, Laine M, Hämäläinen H. The effects of memory load on event-related EEG desynchronization and synchronization. Clin Neurophysiol. 2000;111:2071–2078. doi: 10.1016/S1388-2457(00)00429-6. [DOI] [PubMed] [Google Scholar]
- Lee PS, Chen YS, Hsieh JC, Su TP, Chen LF. Distinct neuronal oscillatory responses between patients with bipolar and unipolar disorders: a magnetoencephalographic study. J Affect Disord. 2010;123:270–275. doi: 10.1016/j.jad.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva FH. A critical review of clinical applications of topographic mapping of brain potentials. J Clin Neurophysiol. 1990;7:535–551. doi: 10.1097/00004691-199010000-00008. [DOI] [PubMed] [Google Scholar]
- Mitchell DJ, McNaughton N, Flanagan D, Kirk IJ. Frontal-midline theta from the perspective of hippocampal “theta”. Prog Neurobiol. 2008;86:156–185. doi: 10.1016/j.pneurobio.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Muir WJ, St Clair DM, Blackwood DH. Long-latency auditory event-related potentials in schizophrenia and in bipolar and unipolar affective disorder. Psychol Med. 1991;21:867–879. doi: 10.1017/S003329170002986X. [DOI] [PubMed] [Google Scholar]
- O’Donnell TO, Rotzinger S, Ulrich M, Hanstock CC, Nakashima TT, Silverstone PH. Effects of chronic lithium and sodium valproate on concentrations of brain amino acids. Eur Neuropsychopharmacol. 2003;13:220–227. doi: 10.1016/S0924-977X(03)00070-1. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Hetrick WP, Vohs JL, Krishnan GP, Carroll CA, Shekhar A. Neural synchronization deficits to auditory stimulation in bipolar disorder. NeuroReport. 2004;15:1369–1372. doi: 10.1097/01.wnr.0000127348.64681.b2. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Vohs JL, Hetrick WP, Carroll CA, Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. Int J Psychophysiol. 2004;53:45–55. doi: 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. Neuroimage. 2005;27:341–356. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Oribe N, Onitsuka T, Hirano S, Hirano Y, Maekawa T, Obayashi C, Ueno T, Kasai K, Kanba S. Differentiation between bipolar disorder and schizophrenia revealed by neural oscillation to speech sounds: an MEG study. Bipolar Disord. 2010;12:804–812. doi: 10.1111/j.1399-5618.2010.00876.x. [DOI] [PubMed] [Google Scholar]
- Özerdem A, Güntekin B, Tunca Z, Başar E. Brain oscillatory responses in patients with bipolar disorder manic episode before and after valproate treatment. Brain Res. 2008;1235:98–108. doi: 10.1016/j.brainres.2008.06.101. [DOI] [PubMed] [Google Scholar]
- Özerdem A, Güntekin B, Saatçi E, Tunca Z, Başar E. Disturbance in long distance gamma coherence in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:861–865. doi: 10.1016/j.pnpbp.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Özerdem A, Güntekin B, Atagün I, Turp B, Başar E. Reduced long distance gamma (28–48 Hz) coherence in euthymic patients with bipolar disorder. J Affect Disord. 2011;132:325–332. doi: 10.1016/j.jad.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Pantev C, Roberts LE, Elbert T, Ross B, Wienbruch C. Tonotopic organization of the sources of human auditory steady-state responses. Hear Res. 1996;101:62–74. doi: 10.1016/S0378-5955(96)00133-5. [DOI] [PubMed] [Google Scholar]
- Paquette C, Bourassa M, Peretz I. Left-ear advantage in pitch perception of complex tones without energy at the fundamental frequency. Neuropsychologia. 1996;34:153–157. doi: 10.1016/0028-3932(95)00095-X. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane RD. The neurobiology of emotion perception I: towards an understanding of the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/S0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rass O, Krishnan G, Brenner CA, Hetrick WP, Merrill CC, Shekhar A, O’Donnell BF. Auditory steady state response in bipolar disorder: relation to clinical state, cognitive performance, medication status, and substance disorders. Bipolar Disord. 2010;12:793–803. doi: 10.1111/j.1399-5618.2010.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, Moore PB. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006;93:105–115. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Sakowitz OW, Schürmann M, Başar E. Oscillatory frontal theta responses are increased upon bisensory stimulation. Clin Neurophysiol. 2000;111:884–893. doi: 10.1016/S1388-2457(99)00315-6. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, Sherwood AR, Fischer IA, Yurgelun-Todd DA, Tohen M, McCarley RW. First-episode schizophrenic psychosis differs from first-episode affective psychosis and controls in P300 amplitude over left temporal lobe. Arch Gen Psychiatry. 1998;55:173–180. doi: 10.1001/archpsyc.55.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, McCarley RW. P300 topography differs in schizophrenia and manic psychosis. Biol Psychiatry. 1999;45:98–106. doi: 10.1016/S0006-3223(98)00208-X. [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Petsche H, Rappelsberger P, Shaw GL, von Stein A. Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci USA. 1998;95:7092–7096. doi: 10.1073/pnas.95.12.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze KK, Hall MH, McDonald C, Marshall N, Walshe M, Murray RM, Bramon E. P50 Auditory Evoked Potential Suppression in Bipolar Unaffected Relatives. Biol Psychiatry. 2007;62:121–128. doi: 10.1016/j.biopsych.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Schulze KK, Hall MH, McDonald C, Marshall N, Walshe M, Murray RM, Bramon E. Auditory P300 in patients with bipolar disorder and their unaffected relatives. Bipolar Disord. 2008;10:377–386. doi: 10.1111/j.1399-5618.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- Souza VB, Muir WJ, Walker MT, Glabus MF, Roxborough HM, Sharp CW, Dunan JR, Blackwood DH. Auditory P300 event-related potentials and neuropsychological performance in schizophrenia and bipolar affective disorder. Biol Psychiatry. 1995;37:300–310. doi: 10.1016/0006-3223(94)00131-L. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- Strik WK, Fallgatter AJ, Brandeis D, Pascual-Marqui RD. Three-dimensional tomography of event-related potentials during response inhibition: evidence for phasic frontal lobe activation. Electroencephalogr Clin Neurophysiol. 1998;108:406–413. doi: 10.1016/S0168-5597(98)00021-5. [DOI] [PubMed] [Google Scholar]
- von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38:301–313. doi: 10.1016/S0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Yener G, Başar E. Sensory evoked and event related oscillations in Alzheimer’s disease: a short review. Cogn Neurodyn. 2010;4:263–274. doi: 10.1007/s11571-010-9138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yener GG, Güntekin B, Oniz A, Başar E. Increased frontal phase-locking of event-related theta oscillations in Alzheimer patients treated with cholinesterase inhibitors. Int J Psychophysiol. 2007;64:46–52. doi: 10.1016/j.ijpsycho.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ. Neural specializations for tonal processing. Ann N Y Acad Sci. 2001;930:193–210. doi: 10.1111/j.1749-6632.2001.tb05734.x. [DOI] [PubMed] [Google Scholar]