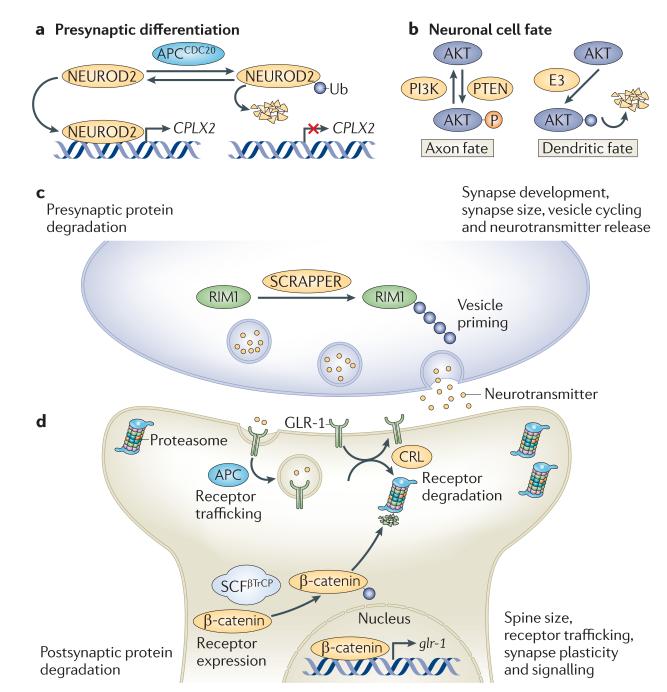
Figure 3. Polarized functions of ubiquitylation in neurons.
Stringent spatial control of the ubiquitin network is crucial for many aspects in the life, death and functionality of the nervous system. a | The ubiquitin E3 ligase anaphase-promoting complex (APC)21 is considered to be a key regulator of presynaptic axonal differentiation. APC and its co-activator CD20 (APCCD20) induce the degradation of the proneuronal transcription factor neurogenic differentiation factor 2 (NEUROD2), which regulates the transcription of complexin II (CPLX2) and thereby suppresses presynaptic differentiation specifically at presynaptic sites. b | The spatially restricted ubiquitylation and subsequent proteasomal degradation of AKT in dendrites underlies the generation of neuronal polarity, during which axonal and dendritic fates are distinguished by high and low levels of phosphoinositide 3-kinase (PI3K) signalling, respectively. To further reinforce high PI3K activation in axons, phosphatase and tensin homologue (PTEN; which antagonizes PI3K signalling) was recently found to be reciprocally downregulated by NEDD4-mediated ubiquitylation in axonal growth cones. c,d | A spatially controlled ubiquitin–proteasome system network is essential for maintaining optimal protein levels and synaptic balance. At the presynaptic side, the turnover of proteins regulating vesicle priming and release, such as DUNC13 in Drosophila melanogaster and RAB3-interacting molecule 1 (RIM1) in mice, are strictly controlled by ubiquitylation. In mice, the E3 responsible for RIM1 ubiquitylation is SCRAPPER (c). In response to neuronal stimulation, active proteasomes are highly enriched in the postsynaptic compartment, where they are crucial for controlling spine size, receptor trafficking, synapse plasticity and signalling downstream of neurotransmitter receptors. In Caenorhabditis elegans, Glu receptor 1 (GLR-1) is regulated by ubiquitylation on many levels, including receptor internalization and trafficking (by the APC) and receptor degradation (by a cullin–RING ubiquitin ligase (CRL) complex made up of cullin 3 and the BTB-Kelch protein KEL-8). Moreover, GLR-1 is also transcriptionally controlled by WNT signalling, which in turn is regulated by the Skp–cullin–F-box–βTrCP (SCFβTrCP)-mediated ubiquitylation and degradation of β-catenin (d). Ub, ubiquitin.