Abstract
Objectives
The antipsychotic drug thioridazine is active in the murine model of tuberculosis infection, which is predominantly intracellular in nature. Recent clinical reports suggest that thioridazine may play a role in the treatment of drug-resistant tuberculosis. We studied the tuberculocidal activity of thioridazine in guinea pigs, which develop necrotic lung granulomas histologically resembling their human counterparts.
Methods
Pharmacokinetic studies were performed in guinea pigs to establish human-equivalent doses of thioridazine. Guinea pigs were aerosol-infected with ∼100 bacilli of Mycobacterium tuberculosis and single-drug treatment was started 4 weeks later with a range of thioridazine doses daily (5 days/week) for up to 4 weeks. Control animals received no treatment or 60 mg/kg isoniazid.
Results
The human-equivalent dose of thioridazine was determined to be 5 mg/kg with saturable absorption noted above 50 mg/kg. At the start of treatment, the lung bacterial burden was ∼6.2 log10 cfu. Although isoniazid reduced bacillary counts more than 10-fold, thioridazine monotherapy showed limited killing over the range of doses tested, reducing lung bacillary counts by 0.3–0.5 log10 following 1 month of treatment. Thioridazine was tolerated up to 40 mg/kg.
Conclusions
Thioridazine has limited bactericidal activity against extracellular bacilli within necrotic granulomas. Its contribution to the sterilizing activity of combination regimens against drug-susceptible and drug-resistant tuberculosis remains to be determined.
Keywords: Mycobacterium tuberculosis, phenothiazine, isoniazid, chemotherapy, toxicity, guinea pigs
Introduction
Strategies involving new uses of existing drugs are urgently needed to reduce the time required to cure patients with drug-susceptible (DS) and multidrug-resistant (MDR) tuberculosis (TB).1 Thioridazine, an old antipsychotic phenothiazine, has been shown to have in vitro activity against DS- and MDR-TB isolates. The in vitro-derived MIC (6–32 mg/L) of thioridazine is significantly higher than the corresponding value within macrophages (0.1 mg/L), since the drug concentrates more than 100-fold in these cells. Thus clinically relevant doses of thioridazine might be highly active against intracellular Mycobacterium tuberculosis without associated systemic toxicity.2 Moreover, the drug is active against M. tuberculosis in a starved state and within phagocytes, and also exhibits synergism with rifampicin and streptomycin.3 Thioridazine disrupts aerobic respiration under microaerophilic conditions by targeting the type II NADH dehydrogenase and succinate dehydrogenase, damaging the cell envelope and inhibiting efflux pumps.4,5 Given its unique mechanisms of action and its apparent efficacy against non-replicating and intracellular M. tuberculosis, thioridazine has been evaluated for the treatment of MDR-TB and extensively drug-resistant (XDR) TB.6,7 In a small, proof-of-concept study, thioridazine was used in combination with linezolid and moxifloxacin to treat XDR-TB in Argentina, as well as in the therapy of terminal XDR-TB patients in India.6,7 However, the tolerability and efficacy of this drug has not been demonstrated convincingly in relevant animal models. This dose-ranging study was undertaken to evaluate the bactericidal activity of thioridazine in the guinea pig model of chronic TB infection, in which the majority of bacilli are located in the extracellular compartment of necrotic lung granulomas.8
Materials and methods
Bacterial strains and growth conditions
M. tuberculosis H37Rv, twice passaged in mice (H37Rv-JHU), was used.9 Prior to aerosol infection, cultures were grown to log phase (optical density at 600 nm of ∼0.6) in Middlebrook 7H9 broth (Difco Laboratories) supplemented with 10% oleic acid/albumin/dextrose/catalase (Becton Dickinson), 0.05% Tween and 0.1% glycerol on a shaker at 37°C.
Animals
Female outbred Hartley guinea pigs (250–300 g) with and without vascular catheters cannulating the jugular vein were purchased from Charles River Labs (Wilmington, MA, USA). All animals were maintained under pathogen-free conditions and fed water and chow ad libitum. All procedures involving animals were performed in compliance with the US Animal Welfare Act regulations and Public Health Service Policy according to protocols approved by the Institutional Animal Care and Use Committee at the Johns Hopkins University (JHU protocol approval number GP12M88).
Pharmacokinetic studies
Thioridazine was purchased from Sigma (St Louis, MO, USA). Separate groups of three guinea pigs were given a single dose (5, 10, 25, 50, 75, 100, 150 or 200 mg/kg) of thioridazine. Doses were prepared in 40% sucrose (w/v) in a final volume of 0.5 mL and were delivered in the posterior oropharynx by an automatic pipette with disposable tip. Blood (∼0.0003 L) was collected without anticoagulation serially from individual guinea pigs through the intravenous catheter at 0.5, 1, 2, 4, 6, 8 and 24 h post-treatment. Serum was separated and stored at −70°C until analysis. Serum thioridazine concentrations were determined by liquid chromatography–mass spectrometry and liquid chromatography–tandem mass spectrometry over the concentration range of 0.005–1 mg/L with dilution to 10 mg/L. Pharmacokinetic variables were calculated from individual thioridazine concentration–time data using non-compartmental methods as implemented in WinNonlin version 5.0 (Pharsight, Mountain View, CA, USA).9–11
Aerosol infections
Guinea pigs were infected with M. tuberculosis H37Rv via the aerosol route using a Madison chamber aerosol generation device (College of Engineering Shops, University of Wisconsin, Madison, WI, USA) calibrated to deliver ∼100 bacilli per guinea pig lung.8 Four animals were sacrificed on the day after infection and 4 weeks later (day −27 and day 0, respectively, relative to treatment initiation) in order to determine the implantation dose and bacillary burden at the start of therapy, respectively.
Antibiotic therapy and study endpoints
Oral therapy with increasing doses of thioridazine or isoniazid (60 mg/kg) administered five times weekly was initiated 1 month after infection [day 0; Table S1 (available as Supplementary data at JAC Online)]. The negative control group received no treatment. Four animals per group were sacrificed after 1 month of antibiotic therapy. Animal body weights were recorded on a weekly basis and lung and spleen weights were recorded at the time of necropsy. Lungs were examined grossly for visible lesions, and small, randomly selected sections were formalin fixed for histopathology. The remainder of each lung was homogenized in 0.01 L of PBS. Lung homogenates were plated on 7H11 plates containing cycloheximide (50 mg/L), carbenicillin (100 mg/L), polymyxin B (200 000 U/L) and trimethoprim (20 mg/L) and incubated for 28 days at 37°C for cfu enumeration. In addition, undiluted and diluted lung homogenates were plated on thioridazine-containing (4 × MIC = 40 mg/L) 7H11 plates in order to quantify the number of thioridazine-resistant colonies.
Statistical analysis
Pharmacokinetic parameters were summarized using descriptive statistics. The cfu data were derived from four guinea pigs per group. Log-transformed cfus were used to calculate means and standard deviations. Comparisons of data among experimental groups were performed by analysis of variance followed by Bonferroni multiple comparison tests using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). Bonferroni multiple comparison tests at an overall alpha of 0.05 were considered to be statistically significant.
Results
Identification of the human-equivalent dose of thioridazine in guinea pigs
Based on the area under the serum concentration–time curve from 0 h to infinity (AUC0–∞), the human-equivalent dose of isoniazid in guinea pigs was determined previously to be 60 mg/kg.9 In the current study, we determined the human-equivalent dose of thioridazine [Table 1 and Figure S1 (available as Supplementary data at JAC Online)].12 Since pharmacodynamic data are not available to permit characterization of the anti-TB activity of thioridazine as concentration dependent or time dependent, the AUC0–∞ value, which directly reflects each of these different types of activities, was selected to model drug exposures in human plasma in this study (Table 1). After oral administration of thioridazine in guinea pigs, the peak serum concentrations (Cmax) and total exposure (AUC0–∞) were linear to 50 mg/kg, after which saturable absorption was noted. A dose of 5 mg/kg was determined to be equivalent to the human exposure.
Table 1.
Single-dose pharmacokinetics of thioridazine in guinea pigs and humans
| Test species | Drug dosage | Cmax (ng/mL) | Tmax (h) | t1/2 (h) | AUC0–∞ (ng·h/mL) |
|---|---|---|---|---|---|
| Guinea pig | 5 mg/kg | 404 ± 155 | 1.5 ± 0.7 | 3.2 ± 1.4 | 2188 ± 10 |
| Guinea pig | 10 mg/kg | 525 ± 165 | 1.5 ± 0.9 | 6.8 ± 2.0 | 3766 ± 1199 |
| Guinea pig | 25 mg/kg | 942 ± 350 | 1.2 ± 0.8 | 6.4 ± 4.3 | 7684 ± 6357 |
| Guinea pig | 50 mg/kg | 1234 ± 225 | 2.2 ± 1.8 | 4.4 ± 0.2 | 11 344 ± 435 |
| Guinea pig | 75 mg/kg | 1366 ± 325 | 1.7 ± 0.6 | 5.3 ± 0.6 | 11 918 ± 1315 |
| Guinea pig | 100 mg/kg | 1270 ± 7 | 1.0 ± 0.0 | 4.6 ± 0.6 | 12 411 ± 325 |
| Guinea pig | 150 mg/kg | 1051 ± 3 | 2.5 ± 2.12 | 8.4 ± 2.0 | 12 399 ± 2625 |
| Guinea pig | 200 mg/kg | 1232 ± 245 | 10.0 ± 0.0 | 9.8 ± 3.2 | 14 887 ± 1505 |
| Human12 | 25 mg | 111 ± 51 | 1.8 ± 0.8 | 6.8 ± 1.7 | 555 ± 276 |
| Human12 | 50 mg | 197 ± 102 | 1.4 ± 0.4 | 8.2 ± 1.5 | 1084 ± 583 |
| Human12 | 100 mg | 372 ± 137 | 1.5 ± 0.4 | 9.3 ± 1.9 | 2639 ± 859 |
Data represent mean ± SD values for three animals.
Safety and tolerability of thioridazine
All four animals receiving 160 mg/kg thioridazine died within 1 week of treatment initiation, but premature mortality was not observed in any of the other groups. Animals treated with 2.5–20 mg/kg thioridazine showed mild weight gain during the 4 weeks of treatment, while those receiving 40 mg/kg thioridazine had minimal weight gain and those receiving 80 mg/kg thioridazine showed mild weight loss (Figure S2, available as Supplementary data at JAC Online).
Organ weights, gross pathology and histology following treatment
Normalized mean lung and spleen weights of treated guinea pigs did not show any significant changes compared with those of the untreated group, although animals treated with 40 mg/kg thioridazine showed a trend toward greater spleen weights, and those treated with 80 mg/kg thioridazine had larger mean lung and spleen weights (Figure S3, available as Supplementary data at JAC Online).
Isoniazid treatment led to a decrease in the number and size of granulomas, which tended to localize more peripherally, whereas no significant differences in the size or number of tubercle lesions or histological findings were observed between any of the thioridazine-treated groups relative to untreated controls (data not shown).
Evaluation of bactericidal activity of thioridazine against chronic TB infection in guinea pigs
A total of 1.6 log10 bacilli were implanted in guinea pig lungs on day −27, and the organisms multiplied to a peak lung burden of 6.2 log10 cfu on day 0. Bacillary growth was controlled in the lungs of untreated guinea pigs, which had 6.2 log10 cfu at the end of the study (Figure 1). Isoniazid showed significant bactericidal activity, reducing lung cfu 10-fold to 5.2 log10 cfu after 28 days of treatment (P < 0.0001). Treatment with thioridazine alone at doses ranging from 2.5 to 80 mg/kg reduced lung cfu counts by 0.3–0.5 log10, although this result was not statistically significant. Thioridazine-resistant mutant colonies were not recovered following 1 month of treatment at any of the doses tested.
Figure 1.
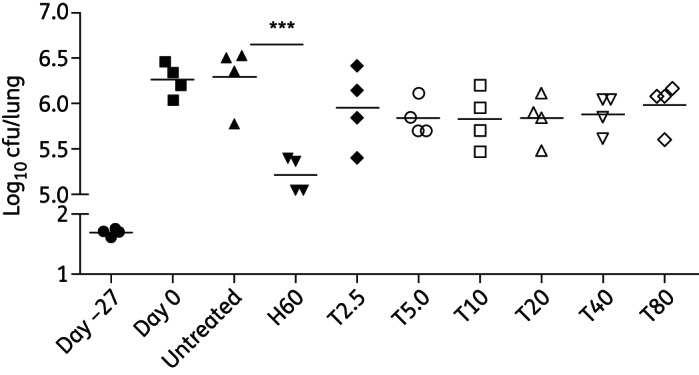
Anti-TB activity of thioridazine in chronically infected guinea pigs. Animals were infected via aerosol with ∼102 cfu of the H37Rv strain and were either left untreated or treated with drugs beginning 1 month after infection. H, isoniazid; T, thioridazine. Numbers after H or T refer to doses (mg/kg). ***P < 0.0001.
Discussion
Recent efforts have focused on developing alternative animal models that more closely approximate TB-related pathology than the mouse, with the goal of more accurately predicting the activity of novel anti-TB drugs and drug combinations in humans.8,9,11 Unlike mice, guinea pigs infected with M. tuberculosis form necrotic granulomas histologically resembling their human counterparts.13 Such lesions in humans and guinea pigs harbour persistent bacilli, which may encounter microenvironmental stress conditions, including hypoxia, that are absent in mice.14–16 Therefore the guinea pig model of chronic TB infection is highly relevant for evaluating the anti-TB activity of novel and repurposed drugs.8,9,11
We found that doses of thioridazine >40 mg/kg are not well tolerated in guinea pigs, and that daily administration of 160 mg/kg of the drug uniformly leads to rapid death. Although the cause of death was not determined in this group, we hypothesize that it may have been related to the well-known cardiac toxicity of thioridazine, which is dose related.17 Monotherapy with human-equivalent doses of thioridazine in guinea pigs did not show dose-dependent activity, and very limited bacillary killing was observed for the range of doses tested. The discrepancy in our findings and those reported in the mouse model may be related to the pharmacokinetic properties of the drug, a difference in drug dosing or routes of drug administration.18,19 Thioridazine is known to concentrate within macrophages, which may favour its activity against the predominantly intracellular TB infection in mice.3,18,19 On the other hand, limited drug penetration into the necrotic cores of guinea pig granulomas may have diminished its activity in this model due to its relatively high protein binding (95% in humans).11,20 These hypotheses can be explored further using C3HeB/FeJ mice, which, like guinea pigs, develop necrotic lung granulomas after infection with M. tuberculosis.21 In addition, future pharmacokinetic studies will focus on measuring plasma concentrations of thioridazine in relevant animal models in order to more accurately reflect human drug exposures.
Thioridazine has been shown to have activity against replicating and non-replicating bacilli, including those with resistance to first-line drugs. Additional animal studies are required to determine whether thioridazine can improve the sterilizing activity of combination regimens against persistent bacilli in vivo, with the goal of shortening the duration of treatment for DS- and MDR-TB.
Funding
This study was supported by R01 AI083125 from theNIH/NIAID to P. C. K. and by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University (NIH grants P30 CA006973 and UL1 RR025005) and the Shared Instrument Grant (1S10RR026824-01). The project described was supported in part by grant number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research.
Transparency declarations
None to declare.
Disclaimer
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Supplementary data
References
- 1.Dutta NK, Karakousis PC. Tuberculosis chemotherapy: present situation, possible solutions, and progress towards a TB-free world. Indian J Med Microbiol. 2012;30:261–3. doi: 10.4103/0255-0857.99481. [DOI] [PubMed] [Google Scholar]
- 2.Ordway D, Viveiros M, Leandro C, et al. Clinical concentrations of thioridazine kill intracellular multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2003;47:917–22. doi: 10.1128/AAC.47.3.917-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viveiros M, Amaral L. Enhancement of antibiotic activity against poly-drug resistant Mycobacterium tuberculosis by phenothiazines. Int J Antimicrob Agents. 2001;17:225–8. doi: 10.1016/s0924-8579(00)00343-5. [DOI] [PubMed] [Google Scholar]
- 4.Dutta NK, Mazumdar K, Dastidar SG, et al. New patentable use of an old neuroleptic compound thioridazine to combat tuberculosis: a gene regulation perspective. Recent Pat Antiinfect Drug Discov. 2011;6:128–38. doi: 10.2174/157489111796064597. [DOI] [PubMed] [Google Scholar]
- 5.Dutta NK, Mehra S, Kaushal D. A Mycobacterium tuberculosis sigma factor network responds to cell-envelope damage by the promising anti-mycobacterial thioridazine. PLoS One. 2010;5:e10069. doi: 10.1371/journal.pone.0010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbate E, Vescovo M, Natiello M, et al. Successful alternative treatment of extensively drug-resistant tuberculosis in Argentina with a combination of linezolid, moxifloxacin and thioridazine. J Antimicrob Chemother. 2012;67:473–7. doi: 10.1093/jac/dkr500. [DOI] [PubMed] [Google Scholar]
- 7.Amaral L, Udwadia Z, Abbate E, et al. The added effect of thioridazine in the treatment of drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2012;16:1706–8. doi: 10.5588/ijtld.12.0616. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad Z, Fraig MM, Bisson GP, et al. Dose-dependent activity of pyrazinamide in animal models of intracellular and extracellular tuberculosis infections. Antimicrob Agents Chemother. 2011;55:1527–32. doi: 10.1128/AAC.01524-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad Z, Klinkenberg LG, Pinn ML, et al. Biphasic kill curve of isoniazid reveals the presence of drug-tolerant, not drug-resistant, Mycobacterium tuberculosis in the guinea pig. J infect Dis. 2009;200:1136–43. doi: 10.1086/605605. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad Z, Nuermberger EL, Tasneen R, et al. Comparison of the ‘Denver regimen’ against acute tuberculosis in the mouse and guinea pig. J Antimicrob Chemother. 2010;65:729–34. doi: 10.1093/jac/dkq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutta NK, Illei PB, Peloquin CA, et al. Rifapentine is not more active than rifampin against chronic tuberculosis in guinea pigs. Antimicrob Agents Chemother. 2012;56:3726–31. doi: 10.1128/AAC.00500-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakraborty BS, Midha KK, McKay G, et al. Single dose kinetics of thioridazine and its two psychoactive metabolites in healthy humans: a dose proportionality study. J Pharm Sci. 1989;78:796–801. doi: 10.1002/jps.2600781003. [DOI] [PubMed] [Google Scholar]
- 13.McMurray DN. Disease model: pulmonary tuberculosis. Trends Mol Med. 2001;7:135–7. doi: 10.1016/s1471-4914(00)01901-8. [DOI] [PubMed] [Google Scholar]
- 14.Vandiviere HM, Loring WE, Melvin I, et al. The treated pulmonary lesion and its tubercle bacillus. II. The death and resurrection. Am J Med Sci. 1956;232:30–7. doi: 10.1097/00000441-195607000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Lenaerts AJ, Hoff D, Aly S, et al. Location of persisting mycobacteria in a guinea pig model of tuberculosis revealed by R207910. Antimicrob Agents Chemother. 2007;51:3338–45. doi: 10.1128/AAC.00276-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haapanen JH, Kass I, Gensini G, et al. Studies on the gaseous content of tuberculous cavities. Am Rev Respir Dis. 1959;80:1–5. doi: 10.1164/arrd.1959.80.1P1.1. [DOI] [PubMed] [Google Scholar]
- 17.Thanacoody RH. Thioridazine: the good and the bad. Recent Pat Antiinfect Drug Discov. 2011;6:92–8. doi: 10.2174/157489111796064588. [DOI] [PubMed] [Google Scholar]
- 18.Martins M, Viveiros M, Kristiansen JE, et al. The curative activity of thioridazine on mice infected with Mycobacterium tuberculosis. In Vivo. 2007;21:771–5. [PubMed] [Google Scholar]
- 19.van Soolingen D, Hernandez-Pando R, Orozco H, et al. The antipsychotic thioridazine shows promising therapeutic activity in a mouse model of multidrug-resistant tuberculosis. PLoS One. 2010;5:e12640. doi: 10.1371/journal.pone.0012640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyberg G, Martensson E. Binding of thioridazine and thioridazine metabolites to serum proteins. An in vitro study. Naunyn Schmiedebergs Arch Pharmacol. 1982;319:189–96. doi: 10.1007/BF00495864. [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal IM, Tasneen R, Peloquin CA, et al. Dose-ranging comparison of rifampin and rifapentine in two pathologically distinct murine models of tuberculosis. Antimicrob Agents Chemother. 2012;56:4331–40. doi: 10.1128/AAC.00912-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


