Summary
Exposure of a developing embryo or fetus to endocrine disrupting chemicals (EDCs) has been hypothesized to increase the propensity of an individual to develop a disease or dysfunction in his/her later life. Although it is important to understand the effects of EDCs on early development in animals, sufficient information about these effects is not available thus far. This is probably because of the technical difficulties in tracing the continuous developmental changes at different stages of mammalian embryos. The zebrafish, an excellent model currently used in developmental biology, provides new insights to the field of toxicological studies. We used the standard whole-mount in situ hybridization screening protocol to determine the early developmental defects in zebrafish embryos exposed to the ubiquitous pollutant, bisphenol A (BPA). Three stages (60–75% epiboly, 8–10 somite, and prim-5) were selected for in situ screening of different molecular markers, whereas BPA exposure altered early dorsoventral (DV) patterning, segmentation, and brain development in zebrafish embryos within 24 hours of exposure.
Key words: Bisphenol A, Embryogenesis, Zebrafish
Introduction
The drastic advancement in industrialization and technology and the growth in human population in the past century have resulted in unprecedented environmental changes in the human history. The production of large amounts of synthetic industrial and biomedical chemicals as well as pollutants poses a risk to our ecosystem and induces negative effects on the health of wildlife and human beings. Some of the more damaging chemical contaminants are classified as endocrine disrupting chemicals (EDCs) because they can interfere with the synthesis, metabolism, and action of endogenous hormones (Phillips et al., 2008; Phillips and Foster, 2008). EDCs exert different biological effects via diverse mechanisms of actions (Judson et al., 2009; Rhind, 2008; Wigle et al., 2008). EDCs are believed to cause damages to human health and the ecological systems. With the emergence of the global problem of chemical contamination, the adverse biological effects of EDCs are gaining attention among the scientific communities, industry, governments, non-governmental organizations, and the public. There is an increasing need for the identification and quantification of all these ubiquitous chemical contaminants. The possible routes of exposure of humans to the EDCs are through the environments, consumer products, and foods (Feron et al., 2002; Mantovani et al., 2006; Poppenga, 2000; Wigle et al., 2008). To safeguard the public health, instrumental chemical analysis has been adopted globally for assessing the risk of human exposure to EDCs and their metabolites (Hotchkiss et al., 2008). Considering the severe long-term impact of EDCs on public health, a sensitive animal model is required to assess the risks of the EDCs for protecting human and ecological health.
Rapid structural and functional changes occur during the fetal life making it a vulnerable period of development. The process of development is not a simple process of unfolding the inherited genetic program, followed by the commitment of cells to specific lineages, and structural and functional differentiation in respective organs/tissues. Developmental plasticity in animals can be influenced by both genomic (epigenetic and genetic) and environmental factors, which leads to considerable changes in the developmental path for adaptive responses in the fetus (Bateson et al., 2004; Gluckman et al., 2009; Gluckman et al., 2008; Gluckman and Hanson, 2007; Gluckman et al., 2007; Gluckman et al., 2005a; Gluckman et al., 2005b; Hanson and Gluckman, 2008). To fill the information gap between exposures to EDCs and the outcomes of developmental failure, an experimental model that enables us to investigate the early developmental stages is essential. Zebrafish has been extensively used in developmental biology and has become an attractive model for chemical screening. This is a highly scalable model with a well-established genome database (Barros et al., 2008; Yeh et al., 2009; Zon and Peterson, 2005). This model has been used in general toxicology studies for decades. General toxicology studies such as identification of the median lethal concentration (LC50) and end-point phenotype have been performed in zebrafish after bisphenol A (BPA) exposure (Duan et al., 2008; McCormick et al., 2011; Saili et al., 2012). Recently, next-generation sequencing technology was used to identify potential genes that are altered after BPA exposure in zebrafish embryos (Lam et al., 2011). However, the unique developmental features of zebrafish have not been used in many studies for characterization of exposure to effect. It is difficult to monitor the effects of EDCs on early development by using mammalian embryos; therefore, zebrafish, which has the ability of external fertilization, is used as an alternative model. Gibert and his colleagues used different in situ molecular markers to examine the developmental stage of otolith formation in zebrafish after BPA exposure (Gibert et al., 2011). Here, we hypothesize that the primary action of EDCs is to prevent normal development during early embryogenesis and cell fate determination (i.e. cell signaling and epigenetic modification) and thus affect normal development (i.e. cell fate determination and organogenesis), which leads to organ dysfunction. In this study, we used the zebrafish model to show that exposure of zebrafish embryos to low doses of BPA caused disturbance in dorsal/ventral patterning and segmentation, which provides a new insight on developmental toxicology of environmental pollutants.
Materials and Methods
Fish strains and maintenance
We used the AB wild-type line in this study. The zebrafish were raised and staged as described previously (Kimmel et al., 1995). All experimental procedures on zebrafish embryos were approved by the Hong Kong Baptist University, Hong Kong Special Administrative Region.
BPA exposure in zebrafish embryos
BPA (Sigma–Aldrich, USA) was dissolved in DMSO and diluted in egg medium (E3 medium). BPA was used at a final concentration of 50 µM in all experiments, which is comparable to the concentrations used in other studies (Lam et al., 2011; Sun et al., 2009). Embryos at 1–4 cell stage were directly exposed to BPA in 2 ml of E3 medium in a 6-well plate. The embryos were grown at 28°C for the selected time points (stages), 8 hours post-fertilization (hpf) (60–75% epiboly), 14 hpf (8–10 somite), 24 hpf (prim-5), and 72 hpf (protruding mouth). Control embryos were treated with equal volume of DMSO as that in the BPA-exposed embryos.
Screening procedure and whole-mount in situ hybridization
We used the whole-mount in situ hybridization (WISH) procedure for screening on the basis of our previous study (Tse and Jiang, 2012). Briefly, BPA-exposed embryos were collected at 3 stages 60–75% epiboly, 8–10 somite (ss), and prim-5 and were fixed in 4% paraformaldehyde (PFA). Standard WISH procedure was applied using zebrafish embryos. Plasmids that were used to make antisense mRNA probes have been published previously: chd (Miller-Bertoglio et al., 1997), eng2 (Schier et al., 1996), eve1 (Joly et al., 1993), gata2 (Detrich et al., 1995), gsc (Stachel et al., 1993), krox20 (Strähle et al., 1993), myoD (Weinberg et al., 1996), pax2a (Krauss et al., 1991), and otx2 (Heisenberg et al., 1996).
Results and Discussion
BPA is one of the most common EDCs, and the chemical properties and toxicities of BPA have been reported. BPA is a selective estrogen receptor modulator (Richter et al., 2007) and can interact with thyroid hormone receptors (Moriyama et al., 2002; Zoeller et al., 2005) and peroxisome proliferator-activated receptors (Riu et al., 2011). At the physiological levels, BPA is suggested to be a factor attributed to the development of metabolic disorders in humans, such as cardiovascular diseases, obesity, and insulin resistance (Polyzos et al., 2012; vom Saal et al., 2012). A considerable number of studies in rodents have reported the negative effects of BPA on the function and development of reproductive and neuronal systems (Jašarević et al., 2011; Wolstenholme et al., 2011; Xi et al., 2011). More importantly, female mice prenatally exposed to BPA showed a decrease in fertility and fecundity (Cabaton et al., 2011) and had an adverse effect on the fertility of the male offspring (Salian et al., 2009). Furthermore, BPA administration in rodents could disturb neurons in the substantia nigra (Tando et al., 2007) and in the hippocampus (Kunz et al., 2011). In previous studies, zebrafish embryos have been exposed to BPA at concentrations similar to those used in this study, and otolith malformations (70 µM) and cardiac edema (65 µM) have been reported (Duan et al., 2008; Gibert et al., 2011). Although the general effects and the effects of BPA on development in rodents and zebrafish have been reported, important information about the effects of BPA in the initial stages of cell development remains to be addressed. To understand the mechanism underlying these effects is important because these data could reveal the fundamental cause of the observed effects; further, the data can be utilized to predict and evaluate the impact of in utero EDC exposure. In this study, we performed screening in the early stage of embryogenesis; we selected 3 critical stages, including dorsoventral (DV) patterning (60–75% epiboly), segmentation (8–10 ss), and brain development (prim-5), within 24 hpf (Tse et al., 2009; Tse et al., 2011).
BPA exposure of embryos at 60–75% epiboly stage disturbs DV patterning
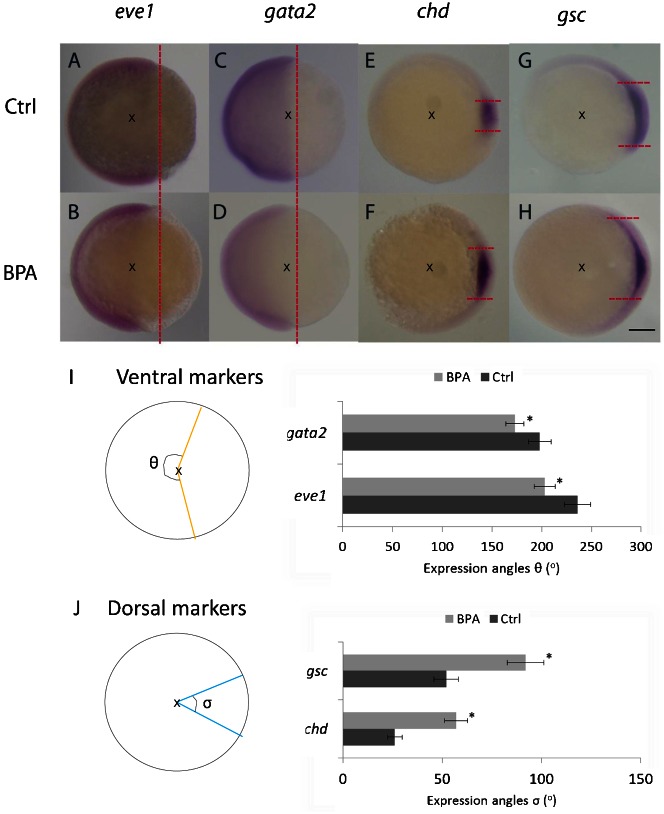
DV patterning is an important developmental process in zebrafish (Schmitz and Campos-Ortega, 1994). Several zebrafish mutants have been identified on the basis of their dorsal or ventral phenotypes, which range from C5 (dorsal) to V4 (ventral) (Mullins et al., 1996; Kishimoto et al., 1997). In this study, we targeted on exposure to BPA at the early development period (within 24 hours). Follow-up examination of the effects of the exposure was performed up to 3 days after fertilization while mild dorsalization (mainly C1–C3) was observed. Dorsalization was characterized by their phenotype of shortened posterior parts during the development (Fig. 1). Because the DV patterning occurs in the early stage of embryogenesis, the effects of the BPA action can be observed by using selected in situ molecular markers (ventral markers, eve1 and gata2; dorsal markers, chd and gsc) at stage of 60–75% epiboly (Tse et al., 2009). Among various validated markers, eve1 is a zebrafish homeobox gene similar to even-skipped in Drosophila (Joly et al., 1993). eve1 is strongly expressed in the ventrolateral marginal cells. The other gene marker gata2 is a hematopoietic transcription factor gene (Detrich et al., 1995) for ventral ectoderm and hematopoietic cells in the ventral mesoderm. To trace the dorsal patterning, we used 2 dorsally expressed markers chordin (chd) and goosecoid (gsc) (Sasai et al., 1995; Stachel et al., 1993). The expression patterns of eve1 and gata2 in embryos exposed to BPA were more restricted in the ventral half of the marginal and the animal zone than that in the controls (Fig. 2A–D). On the other hand, the expression of dorsal markers chd and gsc was greater in the embryos exposed to BPA (Fig. 2E–H). We measured the angles of expressions of the markers (Fig. 2I,J). Taken together, embryos at the 60–75% epiboly stage exposed to BPA showed reduced expression levels of the ventral markers but increased expression levels of the dorsal markers.
Fig. 1. Morphology and phenotypic frequency of 3-day post-fertilized embryos exposed to bisphenol A.
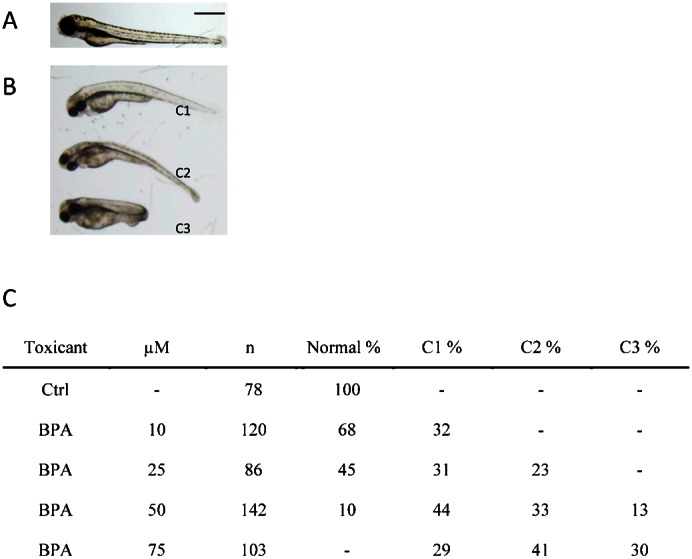
Bisphenol A (BPA)-exposed embryos in an AB wild-type zebrafish showed mild dorsalized phenotypes at 3 days post-fertilization. The control embryos were treated with DMSO (A). BPA exposed embryos showed C1 to C3 mild dorsalization phenotypes (B). Scale bar: 650 µm. Phenotypic frequency is indicated in panel C. C1–C3 phenotypes represent dorsalized phenotype as described (Tse et al., 2009; Mullins et al., 1996). n, number of scored embryos.
Fig. 2. Bisphenol A affects dorsal–ventral patterning at the 60–75% epiboly stage.
Embryos exposed to bisphenol A (BPA) showed narrower expression pattern for the ventral markers eve1 and gata2 (A–D), but wider expression pattern for the dorsal markers chd and gsc (E–H). Red dotted lines indicate the normal expression margin of the ventral markers (ventricle) or dorsal (horizontal) in both BPA-exposed and control embryos. Images were captured in the lateral view (A–D) and animal pole view (E–H), dorsal towards the right in the 60–75% epiboly stage. Scale bar: 250 µm. Schematic diagrams indicate the expression angles of different markers. x marks the center of the embryos, angle of expression of different in situ markers in control and BPA-exposed embryos. θ indicates the angles of the ventral markers (eve1/gata2) with orange lines (I), while σ represents the angles of the dorsal markers (chd/gsc) with blue lines (J). The angles represent the mean of 20 embryos. The expression angles of the ventral markers were smaller (I) but those of the dorsal markers were larger (J), which represents the dorsalization phenotype (*P<0.05).
Exposure of embryos at 8–10 somite stage to BPA affects somatic muscle development
To monitor the trend of altered DV patterning, gata1 and pax2a were used as the markers at 8–10 somite stage. gata1 is ventrally expressed in presumptive hematopoietic cells in 2 lateral stripes (Detrich et al., 1995; Kimmel et al., 1990), while pax2a is used for marking the presumptive neural region (Krauss et al., 1991). The gata1 marker showed widening of the 2 lateral stripes of presumptive hematopoietic cells in BPA-exposed embryos (Fig. 3A,B). Additionally, pax2a staining showed a diffused expression pattern in the mid-hindbrain boundary (mhb). The otic vesicles were missing in the embryos exposed to BPA (Fig. 3C,D). On the other hand, the somite muscle widened in BPA-exposed embryos (Fig. 3E,F). The phenotype was further confirmed by using the myoD somite marker that is expressed in the dorsal mesoderm and somite muscles (Kimmel et al., 1990; Weinberg et al., 1996). Weak and abnormal myoD expression was detected in the BPA-exposed embryos (Fig. 3G,H). In addition to DV patterning, the follow-up in situ experiments illustrated the effects of BPA on somatic muscle formation in the segmentation period.
Fig. 3. Bisphenol A alters somite formation at the 8–10 somite stage.
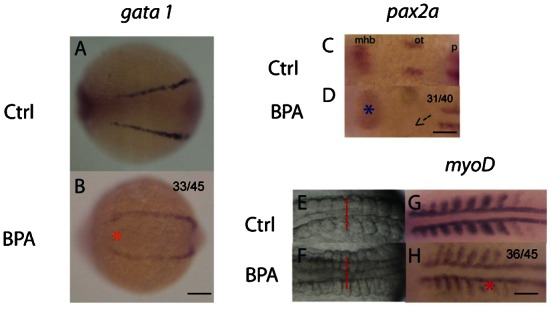
Lateral expansion of the presumptive hematopoietic cell marker (gata 1) was indicated by an orange asterisk, which indicates the dorsalized phenotype in the bisphenol A (BPA)-exposed embryos (A,B). pax2a expression at the 8–10 somite stage, dorsal view (C,D). A blue asterisk indicates abnormal developmental pattern in the mid-hindbrain boundary (mhb) in BPA-exposed embryos. Additionally, an arrow marks the missing of the 2 otic vesicles in BPA-exposed embryos (D). Somite morphology of the control (E) and the BPA-exposed embryos (F) at the 8–10 somite stage. Lateral expansion of somite muscles was observed (red dotted lines). The somite marker, myoD, showed widened and diffused expression in the BPA-exposed embryos (red asterisk) as compared to the control (G,H). mhb, mid-hindbrain boundary; ot, otic vesicle; p, pronephric precursor expression domain. All were head to the left. Scale bars: 75 µm (A,B); 200 µm (C,D); 150 µm (E–H). The number of embryos with the presented phenotype is shown in the top right corner of the panel.
BPA exposure of embryos at the prim-5 stage alters brain development
On the basis of the diffused pax2a expression in the mhb region at the 8–10 somite stage, we suspected that BPA affected brain development during the developmental process. To prove this assumption, the genetic markers krox20, otx2, and eng2b were used to monitor the brain regionalization process at the prim-5 stage (Finkelstein and Boncinelli, 1994; Joyner and Guillemot, 1994; Stuart et al., 1994). Brain regionalization is one of the fundamental processes in the early stages of vertebrate brain development, including the formation of the mhb and its adjacent brain regions (Joyner and Guillemot, 1994). The hindbrain develops into a series of rhombomeres along the anterior–posterior axis of the neural tube. Rhombomeres are believed to be involved in neuronal organization in brain development (Moens and Prince, 2002), while rhombomere 3 (r3) and 5 (r5) could be identified using the transcription factor, krox20 (Oxtoby and Jowett, 1993). In this study, BPA exposure resulted in abnormal and unorganized krox20 expression in the prim-5-stage embryos (Fig. 4A,B). The reduced size of the mhb shown by the eng2b mhb structure marker (Ekker et al., 1992; Fjose et al., 1992) was also found in the BPA-exposed embryos (Fig. 4C,D). These data were consistent with the results of decreased pax2a expression pattern at the mhb region observed in the 8–10 somite stage embryos exposed to BPA (Fig. 3D). To support this observation, an additional marker otx2 was used to confirm if BPA affects the development of midbrain structure (Mercier et al., 1995). The expression of otx2 was decreased in the BPA-exposed embryos, which indicated that the midbrain development was also affected (Fig. 4E,F). The altered percentage was consistent from the early stage to later stages, which suggested the phenotypes might due to the defect in early development. Furthermore, it should be noted that the diffused expression patterns were unlikely to be caused by the developmental delay. Collectively, BPA exposure disturbed the process of brain regionalization, which resulted in the development of abnormal rhombomeres, restricted mhb, and smaller midbrain structure.
Fig. 4. Bisphenol A influences brain development in the prim-5 stage.
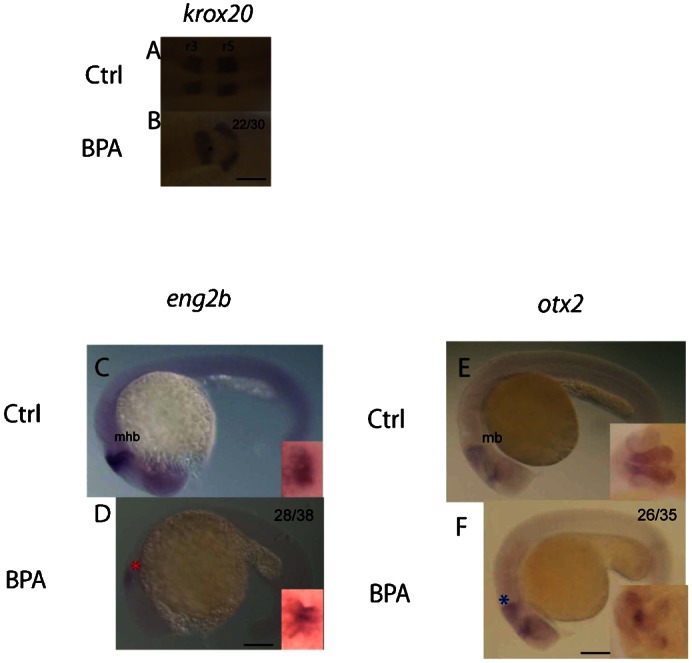
The expression patterns of markers in different brain regions (krox20, eng2b, and otx2) at the prim-5 stage are shown. The expression of krox20, a marker of rhombomeres 3 and 5, dorsal view (A,B). Abnormal patterning of rhombomeres 3 and 5 (black asterisk) was found in BPA-exposed embryos (B), which indicates that regionalization was affected. The patterns of eng2b expression indicated that the mid-hindbrain boundary (mhb) was minimized in the BPA-exposed embryos (red asterisk). Compared to the controls (C), BPA-exposed embryos showed a smaller mhb (D), lateral view; magnified views of the dorsal region are shown in the inserts at the right bottom corner. otx2 expression pattern was restricted in the BPA-exposed embryos (blue asterisk). Compared to the controls (E), the BPA-exposed embryos (F) showed reduced size of the midbrain. Magnified views of the dorsal section are shown in the corner. mb, midbrain; mhb, mid-hindbrain boundary; r3/r5, rhombomere 3/5. All were head to the left. Scale bars: 100 µm (A,B); 125 µm (C–F). The number of embryos with the presented phenotype is shown in the top right corner of the panel.
Although our study does not provide a detailed mechanism of how BPA affects development, it strengthens our understanding about the developmental defect caused by BPA exposure. The resulting phenotype can be caused by complicated crosstalk between signaling pathways. Further studies should be performed to understand how and why the molecular markers listed above were affected. Zebrafish used in this study can act as a screening model to focus the research on the specific time point, organ, and potential signaling pathway involved in development.
Conclusion
In this study, the standard in situ hybridization method was used to examine the effects of EDCs on early embryogenesis. We found that BPA exposure influences DV patterning, somite formation, and brain development in zebrafish embryos. Our study showed the potential use of zebrafish for validating the effects of EDC at a particular developmental stage.
Acknowledgments
This work was supported by the Collaborative Research Fund (HKBU1/CRF/08 to C.K.C.W.), University Grants Committee, Hong Kong. The work in the W.K.F.T. laboratory is supported by the Faculty start-up fund (BIOL3840101).
Footnotes
Competing interests: The authors have no competing interests to declare.
References
- Barros T. P., Alderton W. K., Reynolds H. M., Roach A. G., Berghmans S. (2008). Zebrafish: an emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br. J. Pharmacol. 154, 1400–1413 10.1038/bjp.2008.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P., Barker D., Clutton–Brock T., Deb D., D'Udine B., Foley R. A., Gluckman P., Godfrey K., Kirkwood T., Lahr M. M.et al. (2004). Developmental plasticity and human health. Nature 430, 419–421 10.1038/nature02725 [DOI] [PubMed] [Google Scholar]
- Cabaton N. J., Wadia P. R., Rubin B. S., Zalko D., Schaeberle C. M., Askenase M. H., Gadbois J. L., Tharp A. P., Whitt G. S., Sonnenschein C.et al. (2011). Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environ. Health Perspect. 119, 547–552 10.1289/ehp.1002559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detrich H. W., 3rd, Kieran M. W., Chan F. Y., Barone L. M., Yee K., Rundstadler J. A., Pratt S., Ransom D., Zon L. I. (1995). Intraembryonic hematopoietic cell migration during vertebrate development. Proc. Natl. Acad. Sci. USA 92, 10713–10717 10.1073/pnas.92.23.10713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z., Zhu L., Zhu L., Kun Y., Zhu X. (2008). Individual and joint toxic effects of pentachlorophenol and bisphenol A on the development of zebrafish (Danio rerio) embryo. Ecotoxicol. Environ. Saf. 71, 774–780 10.1016/j.ecoenv.2008.01.021 [DOI] [PubMed] [Google Scholar]
- Ekker M., Wegner J., Akimenko M. A., Westerfield M. (1992). Coordinate embryonic expression of three zebrafish engrailed genes. Development 116, 1001–1010. [DOI] [PubMed] [Google Scholar]
- Feron V. J., Cassee F. R., Groten J. P., van Vliet P. W., van Zorge J. A. (2002). International issues on human health effects of exposure to chemical mixtures. Environ. Health Perspect. 110 Suppl. 6, 893–899 10.1289/ehp.02110s6893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R., Boncinelli E. (1994). From fly head to mammalian forebrain: the story of otd and Otx. Trends Genet. 10, 310–315 10.1016/0168-9525(94)90033-7 [DOI] [PubMed] [Google Scholar]
- Fjose A., Njølstad P. R., Nornes S., Molven A., Krauss S. (1992). Structure and early embryonic expression of the zebrafish engrailed-2 gene. Mech. Dev. 39, 51–62 10.1016/0925-4773(92)90025-F [DOI] [PubMed] [Google Scholar]
- Gibert Y., Sassi–Messai S., Fini J. B., Bernard L., Zalko D., Cravedi J. P., Balaguer P., Andersson–Lendahl M., Demeneix B., Laudet V. (2011). Bisphenol A induces otolith malformations during vertebrate embryogenesis. BMC Dev. Biol. 11, 4 10.1186/1471-213X-11-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P. D., Hanson M. A. (2007). Developmental plasticity and human disease: research directions. J. Intern. Med. 261, 461–471 10.1111/j.1365-2796.2007.01802.x [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Cutfield W., Hofman P., Hanson M. A. (2005a). The fetal, neonatal, and infant environments—the long-term consequences for disease risk. Early Hum. Dev. 81, 51–59 10.1016/j.earlhumdev.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Hanson M. A., Pinal C. (2005b). The developmental origins of adult disease. Matern. Child Nutr. 1, 130–141 10.1111/j.1740-8709.2005.00020.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P. D., Hanson M. A., Beedle A. S. (2007). Early life events and their consequences for later disease: a life history and evolutionary perspective. Am. J. Hum. Biol. 19, 1–19 10.1002/ajhb.20590 [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Hanson M. A., Beedle A. S., Spencer H. G. (2008). Predictive adaptive responses in perspective. Trends Endocrinol. Metab. 19, 109–110, author reply 112 10.1016/j.tem.2008.02.002 [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Hanson M. A., Bateson P., Beedle A. S., Law C. M., Bhutta Z. A., Anokhin K. V., Bougnères P., Chandak G. R., Dasgupta P.et al. (2009). Towards a new developmental synthesis: adaptive developmental plasticity and human disease. Lancet 373, 1654–1657 10.1016/S0140-6736(09)60234-8 [DOI] [PubMed] [Google Scholar]
- Hanson M. A., Gluckman P. D. (2008). Developmental origins of health and disease: new insights. Basic Clin. Pharmacol. Toxicol. 102, 90–93 10.1111/j.1742-7843.2007.00186.x [DOI] [PubMed] [Google Scholar]
- Heisenberg C. P., Brand M., Jiang Y. J., Warga R. M., Beuchle D., van Eeden F. J., Furutani–Seiki M., Granato M., Haffter P., Hammerschmidt M.et al. (1996). Genes involved in forebrain development in the zebrafish, Danio rerio. Development 123, 191–203. [DOI] [PubMed] [Google Scholar]
- Hotchkiss A. K., Rider C. V., Blystone C. R., Wilson V. S., Hartig P. C., Ankley G. T., Foster P. M., Gray C. L., Gray L. E. (2008). Fifteen years after “Wingspread”—environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol. Sci. 105, 235–259 10.1093/toxsci/kfn030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarević E., Sieli P. T., Twellman E. E., Welsh T. H., Jr, Schachtman T. R., Roberts R. M., Geary D. C., Rosenfeld C. S. (2011). Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc. Natl. Acad. Sci. USA 108, 11715–11720 10.1073/pnas.1107958108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly J. S., Joly C., Schulte–Merker S., Boulekbache H., Condamine H. (1993). The ventral and posterior expression of the zebrafish homeobox gene eve1 is perturbed in dorsalized and mutant embryos. Development 119, 1261–1275. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Guillemot F. (1994). Gene targeting and development of the nervous system. Curr. Opin. Neurobiol. 4, 37–42 10.1016/0959-4388(94)90029-9 [DOI] [PubMed] [Google Scholar]
- Judson R., Richard A., Dix D. J., Houck K., Martin M., Kavlock R., Dellarco V., Henry T., Holderman T., Sayre P.et al. (2009). The toxicity data landscape for environmental chemicals. Environ. Health Perspect. 117, 685–695 10.1289/ehp.0800168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Warga R. M., Schilling T. F. (1990). Origin and organization of the zebrafish fate map. Development 108, 581–594. [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- Kishimoto Y., Lee K. H., Zon L., Hammerschmidt M., Schulte–Merker S. (1997). The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development 124, 4457–4466. [DOI] [PubMed] [Google Scholar]
- Krauss S., Johansen T., Korzh V., Fjose A. (1991). Expression of the zebrafish paired box gene pax[zf-b] during early neurogenesis. Development 113, 1193–1206. [DOI] [PubMed] [Google Scholar]
- Kunz N., Camm E. J., Somm E., Lodygensky G., Darbre S., Aubert M. L., Hüppi P. S., Sizonenko S. V., Gruetter R. (2011). Developmental and metabolic brain alterations in rats exposed to bisphenol A during gestation and lactation. Int. J. Dev. Neurosci. 29, 37–43 10.1016/j.ijdevneu.2010.09.009 [DOI] [PubMed] [Google Scholar]
- Lam S. H., Hlaing M. M., Zhang X., Yan C., Duan Z., Zhu L., Ung C. Y., Mathavan S., Ong C. N., Gong Z. (2011). Toxicogenomic and phenotypic analyses of bisphenol-A early-life exposure toxicity in zebrafish. PLoS ONE 6, e28273 10.1371/journal.pone.0028273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Maranghi F., Purificato I., Macrì A. (2006). Assessment of feed additives and contaminants: an essential component of food safety. Ann. Ist. Super. Sanita 42, 427–432. [PubMed] [Google Scholar]
- McCormick J. M., Van Es T., Cooper K. R., White L. A., Häggblom M. M. (2011). Microbially mediated O-methylation of bisphenol A results in metabolites with increased toxicity to the developing zebrafish (Danio rerio) embryo. Environ. Sci. Technol. 45, 6567–6574 10.1021/es200588w [DOI] [PubMed] [Google Scholar]
- Mercier P., Simeone A., Cotelli F., Boncinelli E. (1995). Expression pattern of two otx genes suggests a role in specifying anterior body structures in zebrafish. Int. J. Dev. Biol. 39, 559–573. [PubMed] [Google Scholar]
- Miller–Bertoglio V. E., Fisher S., Sánchez A., Mullins M. C., Halpern M. E. (1997). Differential regulation of chordin expression domains in mutant zebrafish. Dev. Biol. 192, 537–550 10.1006/dbio.1997.8788 [DOI] [PubMed] [Google Scholar]
- Moens C. B., Prince V. E. (2002). Constructing the hindbrain: insights from the zebrafish. Dev. Dyn. 224, 1–17 10.1002/dvdy.10086 [DOI] [PubMed] [Google Scholar]
- Moriyama K., Tagami T., Akamizu T., Usui T., Saijo M., Kanamoto N., Hataya Y., Shimatsu A., Kuzuya H., Nakao K. (2002). Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 87, 5185–5190 10.1210/jc.2002-020209 [DOI] [PubMed] [Google Scholar]
- Mullins M. C., Hammerschmidt M., Kane D. A., Odenthal J., Brand M., van Eeden F. J., Furutani–Seiki M., Granato M., Haffter P., Heisenberg C. P.et al. (1996). Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development 123, 81–93. [DOI] [PubMed] [Google Scholar]
- Oxtoby E., Jowett T. (1993). Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 21, 1087–1095 10.1093/nar/21.5.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips K. P., Foster W. G. (2008). Key developments in endocrine disrupter research and human health. J. Toxicol. Environ. Health B Crit. Rev. 11, 322–344 10.1080/10937400701876194 [DOI] [PubMed] [Google Scholar]
- Phillips K. P., Foster W. G., Leiss W., Sahni V., Karyakina N., Turner M. C., Kacew S., Krewski D. (2008). Assessing and managing risks arising from exposure to endocrine-active chemicals. J. Toxicol. Environ. Health B Crit. Rev. 11, 351–372 10.1080/10937400701876657 [DOI] [PubMed] [Google Scholar]
- Polyzos S. A., Kountouras J., Deretzi G., Zavos C., Mantzoros C. S. (2012). The emerging role of endocrine disruptors in pathogenesis of insulin resistance: a concept implicating nonalcoholic fatty liver disease. Curr. Mol. Med. 12, 68–82 10.2174/156652412798376161 [DOI] [PubMed] [Google Scholar]
- Poppenga R. H. (2000). Current environmental threats to animal health and productivity. Vet. Clin. North Am. Food Anim. Pract. 16, 545–558, viii. [DOI] [PubMed] [Google Scholar]
- Rhind S. M. (2008). Endocrine disruptors and other food-contaminating environmental pollutants as risk factors in animal reproduction. Reprod. Domest. Anim. 43 Suppl. 2, 15–22 10.1111/j.1439-0531.2008.01138.x [DOI] [PubMed] [Google Scholar]
- Richter C. A., Taylor J. A., Ruhlen R. L., Welshons W. V., vom Saal F. S. (2007). Estradiol and bisphenol A stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate mesenchyme cells. Environ. Health Perspect. 115, 902–908 10.1289/ehp.9804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riu A., Grimaldi M., le Maire A., Bey G., Phillips K., Boulahtouf A., Perdu E., Zalko D., Bourguet W., Balaguer P. (2011). Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ. Health Perspect. 119, 1227–1232 10.1289/ehp.1003328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saili K. S., Corvi M. M., Weber D. N., Patel A. U., Das S. R., Przybyla J., Anderson K. A., Tanguay R. L. (2012). Neurodevelopmental low-dose bisphenol A exposure leads to early life-stage hyperactivity and learning deficits in adult zebrafish. Toxicology 291, 83–92 10.1016/j.tox.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salian S., Doshi T., Vanage G. (2009). Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci. 85, 742–752 10.1016/j.lfs.2009.10.004 [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., De Robertis E. M. (1995). Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature 376, 333–336 10.1038/376333a0 [DOI] [PubMed] [Google Scholar]
- Schier A. F., Neuhauss S. C. F., Harvey M., Malicki J., Solnica–Krezel L., Stainier D. Y. R., Zwartkruis F., Abdelilah S., Stemple D. L., Rangini Z.et al. (1996). Mutations affecting the development of the embryonic zebrafish brain. Development 123, 165–178. [DOI] [PubMed] [Google Scholar]
- Schmitz B., Campos–Ortega J. A. (1994). Dorso-ventral polarity of the zebrafish embryo is distinguishable prior to the onset of gastrulation. Roux's Archives of Developmental Biology 203, 374–380 10.1007/BF00188685 [DOI] [PubMed] [Google Scholar]
- Stachel S. E., Grunwald D. J., Myers P. Z. (1993). Lithium perturbation and goosecoid expression identify a dorsal specification pathway in the pregastrula zebrafish. Development 117, 1261–1274. [DOI] [PubMed] [Google Scholar]
- Strähle U., Blader P., Henrique D., Ingham P. W. (1993). Axial, a zebrafish gene expressed along the developing body axis, shows altered expression in cyclops mutant embryos. Genes Dev. 7, 1436–1446 10.1101/gad.7.7b.1436 [DOI] [PubMed] [Google Scholar]
- Stuart E. T., Kioussi C., Gruss P. (1994). Mammalian Pax genes. Annu. Rev. Genet. 28, 219–238 10.1146/annurev.ge.28.120194.001251 [DOI] [PubMed] [Google Scholar]
- Sun H., Shen O.–X., Wang X.–R., Zhou L., Zhen S.–Q., Chen X.–D. (2009). Anti-thyroid hormone activity of bisphenol A, tetrabromobisphenol A and tetrachlorobisphenol A in an improved reporter gene assay. Toxicol. In Vitro 23, 950–954 10.1016/j.tiv.2009.05.004 [DOI] [PubMed] [Google Scholar]
- Tando S., Itoh K., Yaoi T., Ikeda J., Fujiwara Y., Fushiki S. (2007). Effects of pre- and neonatal exposure to bisphenol A on murine brain development. Brain Dev. 29, 352–356 10.1016/j.braindev.2006.10.003 [DOI] [PubMed] [Google Scholar]
- Tse W. K. F., Jiang Y. J. (2012). Functional screen of zebrafish deubiquitylating enzymes by morpholino knockdown and in situ hybridization. Methods Mol. Biol. 815, 321–331 10.1007/978-1-61779-424-7_24 [DOI] [PubMed] [Google Scholar]
- Tse W. K. F., Eisenhaber B., Ho S. H. K., Ng Q., Eisenhaber F., Jiang Y.–J. (2009). Genome-wide loss-of-function analysis of deubiquitylating enzymes for zebrafish development. BMC Genomics 10, 637 10.1186/1471-2164-10-637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse W. K. F., You M.–S., Ho S. H.–K., Jiang Y.–J. (2011). The deubiquitylating enzyme Cops6 regulates different developmental processes during early zebrafish embryogenesis. Int. J. Dev. Biol. 55, 19–24 10.1387/ijdb.103089wt [DOI] [PubMed] [Google Scholar]
- vom Saal F. S., Nagel S. C., Coe B. L., Angle B. M., Taylor J. A. (2012). The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol. Cell. Endocrinol. 354, 74–84 10.1016/j.mce.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. S., Allende M. L., Kelly C. S., Abdelhamid A., Murakami T., Andermann P., Doerre O. G., Grunwald D. J., Riggleman B. (1996). Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development 122, 271–280. [DOI] [PubMed] [Google Scholar]
- Wigle D. T., Arbuckle T. E., Turner M. C., Bérubé A., Yang Q., Liu S., Krewski D. (2008). Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J. Toxicol. Environ. Health B Crit. Rev. 11, 373–517 10.1080/10937400801921320 [DOI] [PubMed] [Google Scholar]
- Wolstenholme J. T., Taylor J. A., Shetty S. R., Edwards M., Connelly J. J., Rissman E. F. (2011). Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS ONE 6, e25448 10.1371/journal.pone.0025448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi W., Lee C. K. F., Yeung W. S. B., Giesy J. P., Wong M. H., Zhang X., Hecker M., Wong C. K. C. (2011). Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus–pituitary–gonadal axis of CD-1 mice. Reprod. Toxicol. 31, 409–417 10.1016/j.reprotox.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Yeh J. R., Munson K. M., Elagib K. E., Goldfarb A. N., Sweetser D. A., Peterson R. T. (2009). Discovering chemical modifiers of oncogene-regulated hematopoietic differentiation. Nat. Chem. Biol. 5, 236–243 10.1038/nchembio.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller R. T., Bansal R., Parris C. (2005). Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology 146, 607–612 10.1210/en.2004-1018 [DOI] [PubMed] [Google Scholar]
- Zon L. I., Peterson R. T. (2005). In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 4, 35–44 10.1038/nrd1606 [DOI] [PubMed] [Google Scholar]