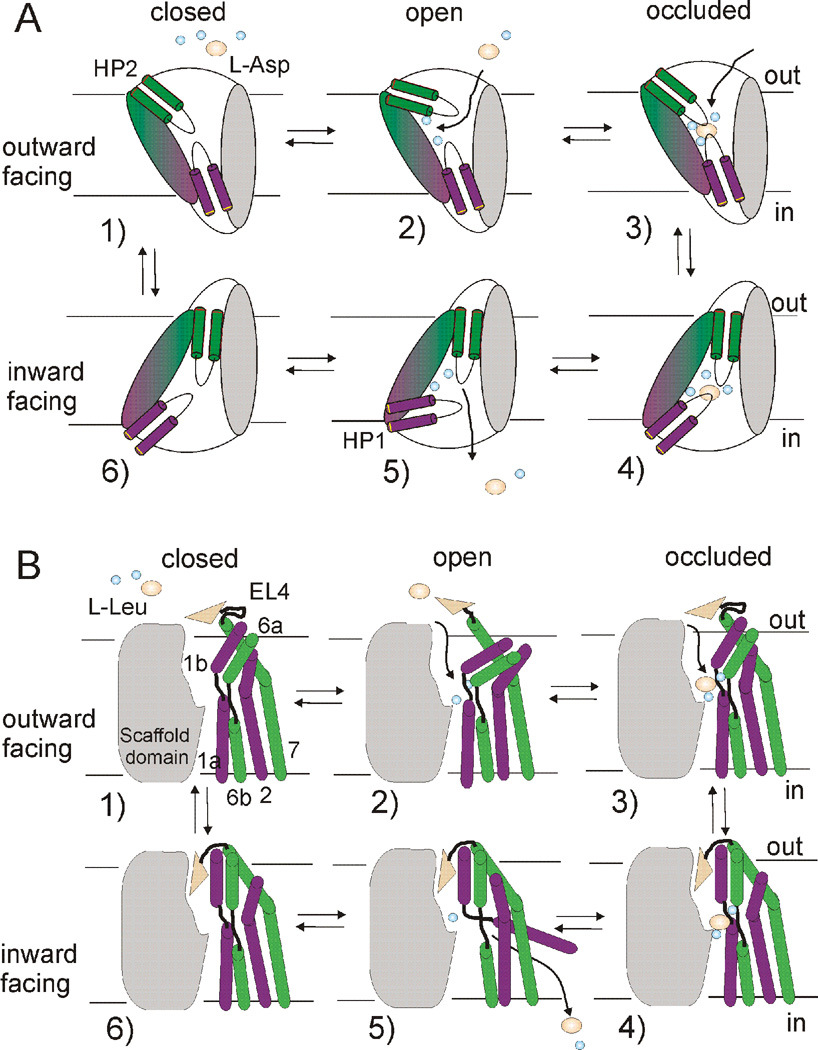
Fig. 8. Models of the transport mechanism in GltPh and LeuT.
In the outward-facing apo state (1) external gate movements (HP2 in GltPh and TMs1b, 6a and EL4 in LeuT) allow for sodium binding, which stabilizes an outward-facing open state (2). This sets up a binding site for substrate and additional ion(s), leading to formation of an outward-occluded state (3). Transition to an inward-facing occluded state (4) involves piston-like motion of the transport domain (magenta and green) relative to the trimerization domain (grey) in GltPh and inwardly-directed movement of EL4 and a coordinated tilt of TMs 1b,6a in LeuT. Release of the first sodium (Na2) leads to opening of the intracellular gate (HP1 in GltPh and TM1a in LeuT) (5) and subsequent release of substrate and additional ion(s). In the inward-facing apo-state (6), the intracellular gate closes to allow for transition to the outward-facing apo-state.