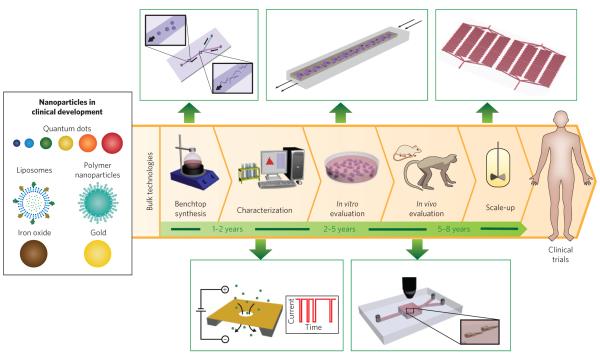
Figure 1. Nanoparticles in clinical development, steps for their translation (with average timescales) and microfluidic methods (green boxes) that could improve or complement current technologies.
Synthesis is carried out in large reaction flasks, whereas microfluidic synthesis is carried out at micro and nano length scales that allow for improved control over reaction conditions. Characterization often involves taking a small sample of nanoparticles and measuring their properties offline, whereas nanopores embedded in microfluidic devices allow for real-time, in-line characterization. In vitro evaluation in plate wells produces a microenvironment far from that in vivo, whereas continuous flow in microfluidic systems result in conditions closer to those in vivo. In vivo evaluation in large animals is helpful for estimating the pharmacology of nanoparticles. To complement these studies microfluidic systems could enable real-time tracking of nanoparticles in large numbers of small organisms. Scale-up is generally carried out in reactor vessels several times larger than benchtop flasks, whereas parallelization of microfluidic channels can increase the production rate of nanoparticles with properties identical to the one at bench scale.