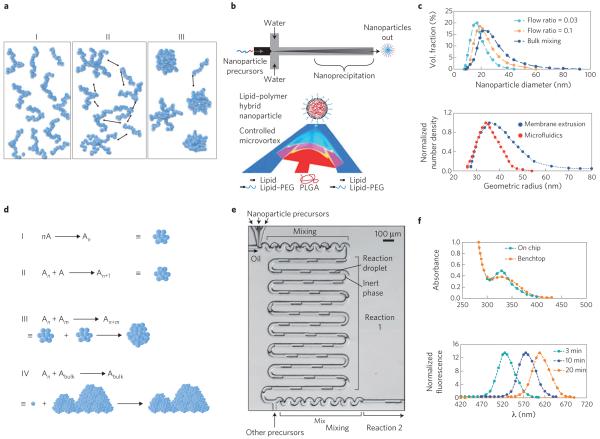
Figure 2. Microfluidic synthesis of nanoparticles.
a, Schematic of the self-assembly mechanism of organic nanoparticles. On mixing with anti-solvent, polymers (or lipids) are brought to the vicinity of each other (I) then nucleate (II), subsequently aggregating into nanoparticles (III). b, Schematic of microfluidic synthesis of organic nanoparticles by rapid mixing through hydrodynamic flow focusing (top) and microvortices (bottom). Red and dark blue indicate organic and aqueous streams, respectively, while pink and light blue indicate their degree of mixing. PEG, polyethylene glycol; PLGA, poly(lactic-co-glycolic acid). c, Size distribution of polymeric nanoparticles (top) and liposomes (bottom) prepared in microfluidics compared with bulk synthesis. In both cases, narrower particle-size distributions are produced through microfluidics. d, Schematic of the self-assembly mechanism of inorganic nanoparticles. Individual molecules first nucleate (I and II), followed by aggregation of nuclei into nanoparticles (III). If the reaction is not quenched or stabilized, nanoparticles tend to agglomerate into bulk material (IV). A refers to individual molecules forming the nanoparticle, and An and Am refer to nuclei formed of n and m number of A molecules, respectively. e, Microfluidic synthesis of inorganic nanoparticles by rapid mixing through two-phase flow where reagents are embedded in fluid droplets carried by an inert fluid. f, Top: sharp versus broad absorption maximum of QDs synthesized in microchannels and bulk, respectively. Bottom: control of the absorption spectra of QDs as function of reaction time. Figure reproduced with permission from: a, ref. 16, © 2003 APS; b, Top: ref. 18, © 2008 ACS; Bottom: ref. 35, © 2012 ACS; c, Top: ref. 18, © 2008 ACS; Bottom: ref. 23, © 2008 Springer; d, ref. 24, © 2005 ACS; e, ref. 27, © 2004 RSC; f, Top: ref. 28, © 2010 Wiley; Bottom: ref. 27, © 2004 RSC.