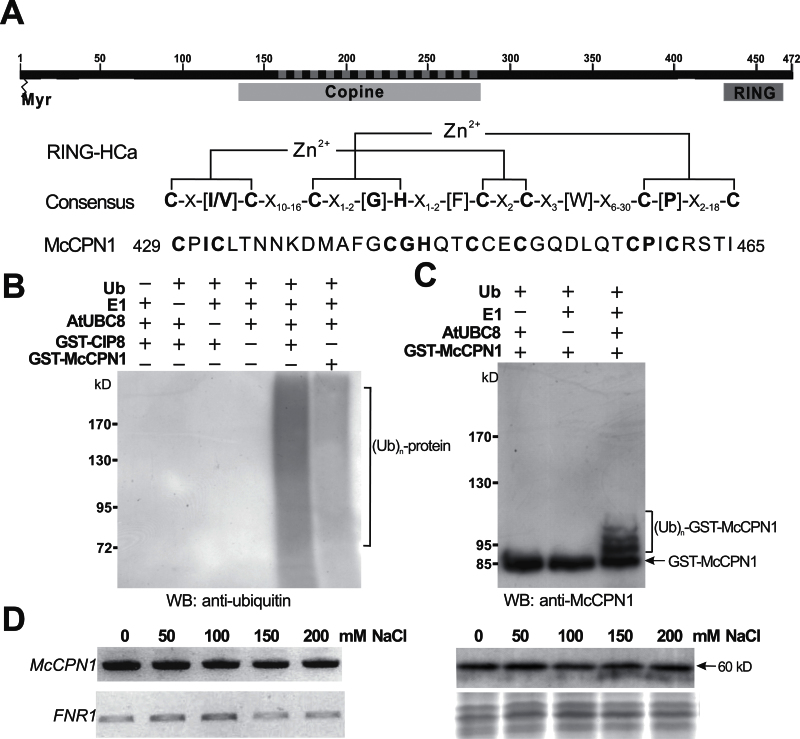
Fig. 1.
Characterization of RING-type E3 ligase McCPN1. (A) Predicted domain structures and RING-HCa consensus sequence of McCPN1. Myr indicates the potential N-myristoylation site, Copine indicates the copine domain, RING indicates the RING domain, and the dashed line indicates the sequences identified from the Y2H screen. Conserved metal ligand binding positions including eight consensus cysteine and histidine residues and zinc (Zn2+) coordinating amino acid pairs are shown in bold. (B) Western blotting using anti-ubiquitin detects protein–ubiquitin conjugates of the in vitro ubiquitination product. In the presence of ubiquitin, E1, and E2 (AtUBC8), both GST–CIP8 and GST–McCPN1 display ubiquitin E3 ligase activity. (C) Western blotting using anti-McCPN1 detects the McCPN1–ubiquitin conjugates, and shifted bands indicate the attachment of 1–3 ubiquitin molecules. The arrow indicates the positions of GST–McCPN1 (85kDa). (D) Gene expression and protein accumulation of McCPN1 in cultured ice plant cells treated with different concentrations of NaCl for 1 week. Left panel: the expression of McCPN1 was analysed by RT–PCR. The expression of FNR1 (ferredoxin-NADP + reductase 1) was used as an internal control. Right panel: the accumulation of McCPN1 was analysed by western blotting using anti-McCPN1 antiserum (top). Coomassie blue-stained total protein was used as a loading control (bottom). The arrow indicates the position of McCPN1 (60kDa).