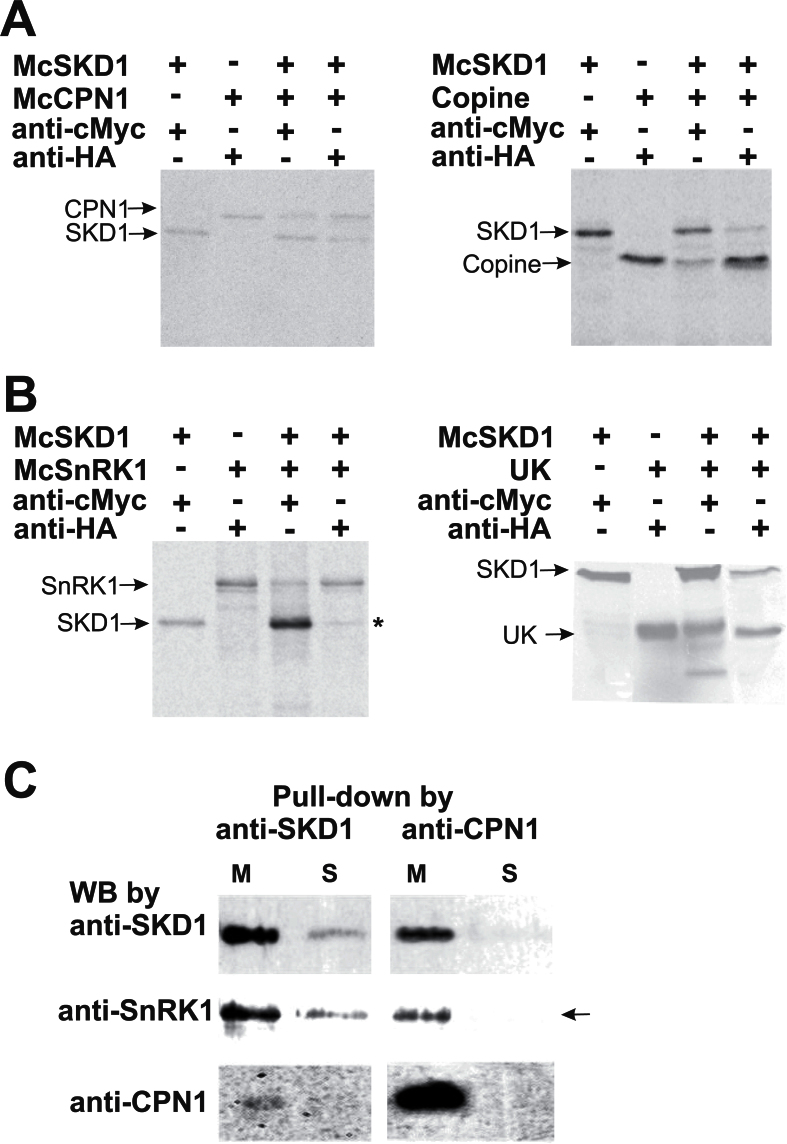
Fig. 3.
Confirmation of McSKD1 interacting with McCPN1 and McSnRK1 by co-immunoprecipitation (Co-IP) and pull-down assay. (A) [35S]Methionine-labelled cMyc-McSKD1, HA-McCPN1, and HA-copine domain were immunoprecipitated with either anti-cMyc or anti-HA antibodies as indicated on the top. The resulting gels were autoradiographed using a PhosphorImager. The positions of the corresponding protein bands are indicated on the left. Left panel: cMyc-McSKD1 co-immunoprecipitated with HA-McCPN1; right panel: cMyc-McSKD1 co-immunoprecipitated with HA-copine domain of McCPN1. The experiments were repeated twice yielding similar results. (B) Co-IP of [35S]methionine-labelled cMyc-McSKD1 and HA-McSnRK1 (left panel) or cMyc-McSKD1 and the HA-UK region of McSnRK1 (right panel). The asterisk indicates a weak McSKD1 band. The experiments were repeated twice yielding similar results. (C) Anti-SKD1 or anti-CPN1 antibodies were first immobilized on the protein A–agarose beads and incubated with the microsomal (M) or soluble (S) protein fractions isolated from salt-stressed ice plant callus. The pull-down protein complex eluted from protein A beads was separated by 10% SDS–PAGE, and western blotting against anti-SKD1, anti-SnRK1, or anti-CPN1 antibodies was performed as indicated. The arrow indicates that McSnRK1 was detected in the pull-down sample using anti-CPN1 in the microsomal fraction but not in the soluble fraction (negative control).