Abstract
Background
Induction of p75 neurotrophin receptor (p75NTR) could be one of the first steps that initiate apoptotic cascade after injury, or it may indicate regeneration responses undertaken by the injured system, possibly in collaboration with resident tropomyosin-receptor-kinase (Trk).
Objective
To measure quantitative changes in messenger RNA (mRNA) expression levels of p75NTR, Trk A, and caspase-9 in rat's injured spinal cord (SCI). The reciprocal interaction between Trk and p75NTR signaling pathways can dictate cellular responses to neurotrophins. p75NTR can regulate Trk-dependent responses, but the role of Trk in regulating p75NTR-dependent signaling is not well documented.
Design
Using real-time polymerase chain reaction, this study analyzed changes in the mRNA abundance of the mentioned genes at 6, 24, and 72 hours and 7 and 10 days after SCI in adult male rats. SCI was induced at T9 level by transsection.
Results
Results show a complicated temporal and spatial pattern of alteration with different degrees and direction (up- or down-regulation) in p75NTR, Trk A, and caspase-9 mRNA expression levels after SCI. The greatest variation was seen in center regions following SCI. This study shows that alteration in p75NTR, Trk A, and caspase-9 expression starts as early as 6 hours after SCI. Alterations in p75NTR, Trk A, and caspase-9 expression within the spinal cord may play a key role in the apoptotic cell death.
Conclusion
Results suggest that the role of p75NTR is to eliminate damaged cells by activating the apoptotic machinery, especially at the center of damage and during first week after injury.
Keywords: p75 neurotrophin receptor (p75NTR), Trk A, Messenger RNA, Apoptosis, Real-time PCR, Spinal cord injuries
Introduction
Great strides have been made over the last decade in understanding of the central nervous system (CNS) responses to traumatic injury. Apoptosis is considered as one of the components of the ‘secondary injury’ cascade that occurs after spinal cord injury (SCI).1 The mechanism of post-traumatic apoptosis after SCI is not well understood. However, it has been hypothesized that the apoptosis occurs after the activation of death receptors such as p75. P75NTR (where NTR is neurotrophin receptor) classically known as the low-affinity nerve growth factor (NGF) receptor is a member of the tumor necrosis factor (TNF) receptor superfamily.2,3
Due to the presence of death domain, cell death, or apoptosis has been attributed as a cellular outcome of p75NTR signaling.4 While p75NTR is able to bind all neurotrophins in the NGF family with equal affinity, ligand binding is not associated with intrinsic enzymatic activity. One of the reasons for the lack of clarity regarding the function of p75NTR is that it does not signal by traditional pathways; it has neither kinase activity nor is it linked to a G-protein-coupled pathway. Instead, this receptor signals by recruiting intracellular adaptor proteins to mediate specific functions.5 In contrast with the tropomyosin-receptor-kinase (Trk) family receptors, the function of p75NTR is still controversial, because this receptor can exert different effects depending on the cellular context, the type of ligand bound to it, and the presence or absence of Trk receptor.6–8 P75NTR is initially expressed very early in embryogenesis in cells derived from all three germinal layers and becomes progressively restricted to specific cell populations such as CNS and peripheral nervous system. The p75NTR expression is induced after pathological conditions in a wide variety of cell types.
A long-standing question in neurotrophin signal transduction is whether heteromeric TrkA–p75NTR complexes possess signaling capabilities that are significantly different from homo-oligomeric TrkA or p75NTR alone.9 An emerging theme in neurotrophin signaling is that reciprocal interaction between Trk and p75NTR signaling pathways can dictate cellular responses to neurotrophins.2,10 Although it is well appreciated that p75NTR can regulate Trk-dependent responses, the role of Trk in regulating p75NTR-dependent signaling is not well documented; nonetheless, it is supported by both biochemical and biological observations.2,7,9 P75NTR-dependent caspases (-3, -6, -8, -9) activation has previously been approved in many in vitro and in vivo studies. It was demonstrated that activation of caspase (-3, -6, and -8) following SCI is dependent on Fas receptor not p75NTR. Moreover, results from Chu and coworkers demonstrated that after SCI, caspase-9 from the intrinsic apoptotic pathway is cleaved through the activation of the p75NTR.1,5,11
This study uses a rat model of transsection SCI to evaluate the gene expression alterations of p75NTR, Trk A, and caspase-9 quantitatively. We analyzed the changes in messenger RNA (mRNA) abundance of these genes at 6 and 24 hours and 3, 7, and 10 days after traumatic injury to rat spinal cord. Our results demonstrate that significant alterations in the spatial-temporal expression of these genes following SCI may involve in survival and death of neural cells after injury.
Experimental procedures
Animals
Fifty-four adult male Wistar rats weighing approximately 180–240 g at the beginning of the experiment were purchased from Ahvaz Jundishapur University of Medical Sciences (Ahvaz, Iran). Forty-five rats received a SCI and nine were kept as control. They were housed in groups (1–6) under a 12:12-hour light/dark cycle with ad libitum access to standard rat chow and water. Rats were housed three per cage to avoid isolation stress. Efforts were made to minimize animal suffering and to reduce the number of animals used. The Animal Ethics Committee for the University of Isfahan approved all experiments.
SCI
Animals were anesthetized with halothane (induction 4%, maintenance 2%, in an oxygen and nitrous oxide 50:50 mixture), a T9 laminectomy was performed exposing a circle of dura, and the spinal cord was injured by bisection. The incisions were closed and rats were returned to their cages after recovering from anesthesia.
Post-surgical care
After SCI, rats were housed three in cage to reduce stress from isolation and kept at 22–25°C on absorbent bedding. The animals were monitored routinely and were given post-operative care on a regular basis. Their bladders were manually expressed twice daily until a reflex bladder was established, usually at 10 days after injury.
Tissue preparation
At the specified times (6 hours, 24 hours, 3, 7, and 10 days) after surgery, the whole spinal cords were rapidly removed from the anesthetized rat (n = 9 for each time point) and placed into ice-cold saline. The dura was removed and around 2.5 cm piece of cord centered at the T9 injury site was cut into three segments: 8-mm tissue centered on the injury epicenter, 8-mm tissue rostral, and 8-mm tissue caudal to the epicenter. Control samples were prepared similarly from the equivalent regions of uninjured rats. The tissue segments were stored at −70°C.
RNA extraction and reverse transcription polymerase chain reaction (RT-PCR)
Total cellular RNA was isolated from frozen tissues using the RNX™ plus solution (CinnaGen, Tehran, Iran). The extracted RNA was dissolved in diethyl pyrocarbonate-treated water. The purity and integrity of the extracted RNA were evaluated by optical density measurements (260/280 nm ratios) and by visual observation of samples electrophoresed on agarose gels. Both methods indicated integrity of the extracted RNA with little or no protein contamination. Complementary DNA synthesis reactions were performed using 1 µg DNase (Fermentas, Glen Burine, Maryland, USA) treated total RNA from each sample and cDNA synthesis kit (Fermentas) with random hexamer (Fermentas) priming in a 20-μl reaction according to the manufacturer's instructions.
Real-time quantitative polymerase chain reaction
Real-time polymerase chain reaction (RT-PCR) was performed in the Chromo4 Detection System (Bio-Rad Laboratories, Hercules, CA, USA). Briefly, 20 ng of cDNA and gene-specific primers were added to SYBR green master kit (Takara, Tokyo, Japan), and subjected to PCR amplification (1 cycle at 95°C for 30 seconds and 45 cycles at 94°C for 5 seconds, 56°C for 20 seconds and 72°C for 15 seconds). All PCR reactions were run in duplicate. The amplified transcripts were quantified using the comparative Ct method (http://www.pebiodocs.com/pebiodocs/04303859.pdf).
Gene-specific primers designed by Beacon Designer 7.5 software. The primers used for RT-PCR were
p75NTR: 5′-AGGGATGGCGTGACTTTC-3′/5′-GTTGGCTTCAGGCTTATGC-3′.
TrkA: 5′-AAGATGCTGGTGGCTGT-3′/5′-CGATGTGTTGGTGCTGTAG-3′.
Caspase-9: 5′-CCACTGCCTCATCATCAAC-3′/5′-CGCCATCTCCATCAAAGC-3′.
Beta-tubulin: 5′-AAGAGGATTTCGGAGAGG-3′/5′-GAACAAAAACAGGACAGAGG-3′.
Expression levels were normalized to that of beta-tubulin. Relative expression data were quantified using 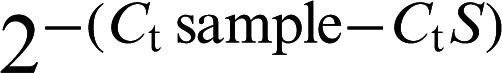 where Ct is the cycle threshold. Relative standard curves were generated by plotting the threshold value (Ct) versus the log of the amount of total cDNA added to the reaction and used to check the efficiency of primers. Calculation of Ct, standard curve preparation, and quantification of mRNA in the samples were performed by software provided with the Chromo4 (option 3). All target genes were normalized to the beta-tubulin housekeeping gene.
where Ct is the cycle threshold. Relative standard curves were generated by plotting the threshold value (Ct) versus the log of the amount of total cDNA added to the reaction and used to check the efficiency of primers. Calculation of Ct, standard curve preparation, and quantification of mRNA in the samples were performed by software provided with the Chromo4 (option 3). All target genes were normalized to the beta-tubulin housekeeping gene.
Statistical analysis
To determine significance, all data were subjected to statistical analysis using a computerized statistics program (Graphpad Prism Software, San Diego, CA, USA). One-way analysis of variance (ANOVA) was used, as indicated in the figure legends, followed by a post hoc test (Tukey) of differences between specific time points. All data are presented as the means ± SEM, when appropriate. A level of P < 0.05 was considered significant.
Results
p75NTR, Trk A, and caspase-9 genes showed significant alterations in mRNA expression after SCI. Fig. 1 shows the temporal changes in mRNA expression of p75NTR, Trk A, and caspase-9 in the rostral, epicenter, and caudal samples after SCI. In each case, the data were expressed as a ratio to that expressed in the same region of control spinal cord.
Figure 1.
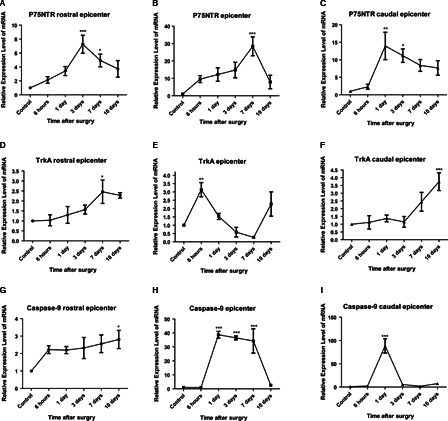
Quantitative RT-PCR analysis of p75 (A–C), TrkA (D–F), and caspase-9 (G–I) spatial-temporal mRNA expression in spinal cord after transsection of the spinal cord in adult rats. The p75NTR and caspase-9 simultaneously increased by 7 days after injury. Contrary to those, the expression levels of TrkA declined by the same time and location. Expression of understudied genes simultaneously elevated at rostral epicenter. Expression pattern of genes at caudal epicenter of injury site was dissimilar and irregular. Error bars show mean ± SD. For each experiment, n = 6 for each subunit, ***P < 0.001, **P < 0.01, *P < 0.05.
Temporal and spatial alterations in p75NTR expression after SCI
P75NTR expression was altered after SCI (Fig. 1 A–C). One-way ANOVA indicated significant effects of both time and location after SCI and of the interaction between these factors. In tissue rostral to the injury site, p75NTR mRNA was increased by 2.13 ± 0.5-fold at 6 hours and 3.41 ± 0.6, 7.25 ± 1.3- fold at 1 and 3 days, respectively, then returned to near control level by 7 (4.93 ± 0.9) and 10 days (3.73 ± 1.2). Only alterations at day 3 and 7 after injury were significant (P < 0.001 and <0.05, respectively) (Fig. 1A). In the epicenter tissue, p75NTR mRNA began to increase 6 hours (9.59 ± 1.9) after SCI, and then continued to increase to 12.18 ± 3.8-fold at 24 hours, 14.78 ± 4.5, 28.42 ± 5.4-fold at 3 and 7 days and reduced to 7.91 ± 3.8-fold at 10 days after injury. Enhancement at days 7 was significant (P < 0.001) (Fig. 1B). The effect of SCI on p75NTR expression was less in caudal tissue with a significant increase at 1 day (13.94 ± 3.9, P < 0.01) and 3 days (11.16 ± 1.9, P < 0.05) after injury (Fig. 1C). Thus, the pattern of p75NTR mRNA increase resembled a wave travelling rostrally – caudally.
Temporal and spatial alterations in Trk A expression after SCI
A very different pattern was seen for the expression of Trk A after SCI (Fig. 1D–F). Trk A mRNA was significantly (P < 0.05) increased by 2.45 ± 0.58 and 3.13 ± 0.4-fold at 7 days and 6 hours in rostral and epicenter tissue, respectively, and decreased in epicenter tissue and increased again in 10 days after injury. In caudal tissue, it was increased continuously.
Temporal and spatial alterations in caspase-9 expression after SCI
In contrast, caspase-9 mRNA (Fig. 1G–I) was higher than normal in epicenter regions at 1, 3, and 7 days. It was rapidly and profoundly increased at the caudal epicenter at 24 hours after SCI and then increased to above normal levels by 3 days. In the rostral part of the wound, there was a profound increase at 1 day followed by an increase at days 3, 7, and 10. The greatest alterations were seen in the epicenter tissue where caspase-9 mRNA increased to more than 40 times the normal level at 1, 3, and 7 days after SCI. At 10 days, epicenter and caudal to the epicenter tissue regions had mRNA about the normal levels (Fig. 1H and I). At day 10 in rostral region the mRNA expression level of caspase-9 was significantly (P < 0.05) more than the normal level.
Discussion
We analyzed expression of the mRNA for the p75NTR, Trk A, and caspase-9 genes after SCI.
To our knowledge, this is the first time that a complex temporal and spatial pattern of altered mRNA expression levels after SCI for p75NTR, Trk A, and caspase-9 genes has been shown. RT-PCR allowed us to obtain quantitative data at different times over the first 10 days after injury and in different regions with respect to the injury epicenter. Each of the p75NTR, Trk A, and caspase-9 genes appeared unique in its temporal-spatial pattern of alterations after SCI. P75NTR expression is induced as a result of a wide variety of neural pathological conditions.12,13 In contrast to the Trk family receptors, the function of p75NTR is still controversial, because p75NTR may exert both pro-survival and pro-apoptotic effects depending on cell type, form of ligand, and the presence or absence of Trks.7 P75NTR has been shown to activate the nuclear factor-kappaB, Akt, also known as Protein Kinase B (PKB), and c-Jun NH2-terminal kinase (JNK) pathways and interacts with several adaptor proteins. Of these, NRAGE (also known as Maged1, Dlxin), NADE (p75NTR-associated cell death executor), and neurotrophin receptor interacting factor (NRIF) have been associated with the induction of apoptosis, and FAP-1, RIP2, and TRAF6 appear to promote cellular survival.5 The interaction of Trks with the neurotrophin receptor p75NTR can induce cell survival or apoptosis. However, Trk A is neuroprotective in several cell lines.14 Furthermore, neurons expressing p75 without co-expressing Trk underwent apoptosis upon NGF treatment.5,15 Some significant data are reported in the previously published studies that p75NTR can cause apoptosis in various neuronal populations by mere high expression or as a mediator of a (pro)neurotrophin death signal.16 Evidence from other studies suggests that p75NTR expression is enhanced post-injury.6,8,17
Most of these studies investigated mRNA expression rather than protein expression with partial chronological resolution. The main function of p75NTR however, remains elusive. With respect to reported data and for revelation of p75NTR role following SCI, we quantitatively examined spatial-temporal alteration of mRNA expression of p75NTR. Severe up-regulation of p75NTR simultaneously with down-regulation of Trk A, especially at the epicenter of injury site may suggest an apoptotic role of p75NTR following SCI. To confirm pro-apoptotic role of p75NTR, therefore, we evaluated mRNA expression of caspase-9. Interestingly, we observed sharp increase of caspase-9 expression with 1-day delay. Our data with caspase-9 strongly support the findings of Chu et al. P75NTR has been hypothesized to play a pro-survival, a pro-apoptotic, and an anti-regeneration role in numerous studies. The results of these researches reflect the complexity of the p75NTR and effect of different factors on functionality of this receptor. Together, our results suggest a possible role for p75NTR conceivably by eliminating of damaged cells through activating the apoptotic machinery, especially at the epicenter of injury site and in first week after injury.
Conclusion
In summary, our results suggest that the p75NTR may play a key role in a very different way at inferior and superior of injury site. The mRNA expression level of p75NTR started to decrease at inferior injury site after 24 hours, whereas expression level of Trk A gradually reached the same level after 72 hours. Our data show that there is an early onset of p75NTR expression and minimal change in Trk A over the same time point. This may lead to the formation of p75NTR–Trk A complex at this location, which can promote the survival of neural cells. The reduction of caspase-9 expression to control level after first day supports this conclusion. P75NTR has formerly been shown to promote cell survival either in association with Trk A or by itself.4,7,11 At the superior of injury site the expression of understudied genes simultaneously elevated (p75NTR by 72 hours, Trk A by 7 days, and caspase-9 by 10 days, Fig. 1). This suggests that neural cells may undergo apoptosis early in injury to offset SCI. Then damaged cells can recover themselves and restore their functions.
mRNA may not necessarily translate to a similar difference at the protein level. However, it is shown that p75NTR appears not to have an undoubtedly distinct role following SCI but instead is a ‘modifier’ protein that is necessary for the maintenance of the balance between survival and death depending on the ratio of pro-natriuretic peptides (NTs) to mature NTs,6 recruiting diverse intracellular-binding proteins and co-receptors,18 the type of injury,12 the cell type affected,10,12,15,17 and the location from the site of the injury.1 This may explain alteration of mRNA expression of p75 in our study. Although the effect of p75NTR is controversial, a homeostatic balance between Trk A and p75NTR is one of the hopeful strategies with a potential for the treatment of SCI.19 Furthermore, the exogenous administration of NTs in SCI models is further challenged by the lack of Trk receptors expression around the injured site. Together, these data suggest that administration of exogenous NTs at appropriate site and time following SCI could help in recovery following SCI. Since the mammalian CNS has a very partial capability to replace neurons that are lost following various injuries, transplantation of neural-like cells derived from embryonic, and/or adult stem cells has been conducted as a potential treatment, recently.10 Unfortunately, the success of the transplantation therapy has not been encouraging so far, because transplanted cells undergo apoptosis.3,10,20 Thus, recognizing involved mechanisms in neuronal survival and death and manipulating the expression and function of related genes is of critical importance to achieve successful transplantation therapy. One of the mechanisms regulating apoptosis of transplanted cells could be related to p75NTR-dependent signaling pathways.
Acknowledgement
This study was supported by grants from the University of Isfahan.
References
- 1.Chu GK, Yu W, Fehlings MG. The p75 neurotrophin receptor is essential for neuronal cell survival and improvement of functional recovery after spinal cord injury. Neuroscience 2007;148(3):668–82 [DOI] [PubMed] [Google Scholar]
- 2.Rankin SL, Guy CS, Mearow KM. TrkA NGF receptor plays a role in the modulation of p75NTR expression. Neurosci Lett 2005;383(3):305–10 [DOI] [PubMed] [Google Scholar]
- 3.Yla-Kotola TM, Kauhanen MS, Asko-Seljavaara SL, Haglund CH, Tukiainen E, Leivo IV. P75 nerve growth factor receptor is expressed in regenerating human nerve grafts. J Surg Res 2008;146(2):254–61 [DOI] [PubMed] [Google Scholar]
- 4.Mamidipudi V, Wooten MW. Dual role for p75(NTR) signaling in survival and cell death: can intracellular mediators provide an explanation? J Neurosci Res 2002;68(4):373–84 [DOI] [PubMed] [Google Scholar]
- 5.Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol 2002;67(3):203–33 [DOI] [PubMed] [Google Scholar]
- 6.Sieck GC, Mantilla CB. Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respir Physiol Neurobiol 2009;169(2):218–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrone L, Paladino S, Mazzone M, Nitsch L, Gulisano M, Zurzolo C. Functional interaction between p75NTR and TrkA: the endocytic trafficking of p75NTR is driven by TrkA and regulates TrkA-mediated signalling. Biochem J 2005;385(Pt 1):233–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajebrahimi Z, Mowla SJ, Movahedin M, Tavallaei M. Gene expression alterations of neurotrophins, their receptors and prohormone convertases in a rat model of spinal cord contusion. Neurosci Lett 2008;441(3):261–6 [DOI] [PubMed] [Google Scholar]
- 9.Lad SP, Peterson DA, Bradshaw RA, Neet KE. Individual and combined effects of TrkA and p75NTR nerve growth factor receptors. A role for the high affinity receptor site. J Biol Chem 2003;278(27):24808–17 [DOI] [PubMed] [Google Scholar]
- 10.Marandi M, Mowla SJ, Tavallaei M, Yaghoobi MM, Jafarnejad SM. Proprotein convertases 1 and 2 (PC1 and PC2) are expressed in neurally differentiated rat bone marrow stromal stem cells (BMSCs). Neurosci Lett 2007;420(3):198–203 [DOI] [PubMed] [Google Scholar]
- 11.Feng D, Kim T, Ozkan E, Light M, Torkin R, Teng KK, et al. Molecular and structural insight into proNGF engagement of p75NTR and sortilin. J Mol Biol 2010;396(4):967–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cragnolini AB, Friedman WJ. The function of p75NTR in glia. Trends Neurosci 2008;31(2):99–104 [DOI] [PubMed] [Google Scholar]
- 13.Haase G, Pettmann B, Raoul C, Henderson CE. Signaling by death receptors in the nervous system. Curr Opin Neurobiol 2008;18(3):284–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman WJ. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci 2000;20(17):6340–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diarra A, Geetha T, Potter P, Babu JR. Signaling of the neurotrophin receptor p75 in relation to Alzheimer's disease. Biochem Biophys Res Commun 2009;390(3):352–6 [DOI] [PubMed] [Google Scholar]
- 16.Alavian KN, Sgado P, Alberi L, Subramaniam S, Simon HH. Elevated P75NTR expression causes death of engrailed-deficient midbrain dopaminergic neurons by Erk1/2 suppression. Neural Dev 2009;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YQ, Bian GL, Bai Y, Cao R, Chen LW. Identification and kainic acid-induced up-regulation of low-affinity p75 neurotrophin receptor (p75NTR) in the nigral dopamine neurons of adult rats. Neurochem Int 2008;53(3–4):56–62 [DOI] [PubMed] [Google Scholar]
- 18.Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, et al. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron 2002;36(3):375–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Zou J, Yao Z, Yu J, Wang H, Xu J. Tetravalent Abeta1–15 vaccine reduces TCR-positive cell infiltration and up-regulates p75 in Tg2576 brains compared to Abeta42 vaccine. J Neuroimmunol February 2010;26;219(1–2):8–16 [DOI] [PubMed] [Google Scholar]
- 20.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science 2001;294(5548):1945–8 [DOI] [PubMed] [Google Scholar]


