Figure 3. Regulation of Sirt1 activity by small molecules.
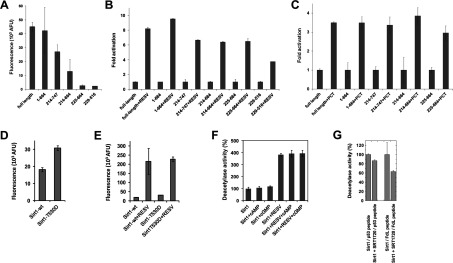
(A) Deacetylation activity of full-length Sirt1 and Sirt1 deletion constructs in the FdL assay. (B) Activation of Sirt1 and Sirt1 deletion constructs in the FdL assay by 100 μM resveratrol (RESV) shows only a weak decrease in the relative stimulation for shortened constructs. (C) Activity in FdL assays of full length Sirt1 and deletion constructs in the presence of 100 μM of the resveratrol-related compound piceatannol (PCT), relative to their basal activity. (D) FdL activity of the phosphorylation mimicking variant Sirt1-T530D compared with the activity of the same amount of wild-type (wt) enzyme. (E) Comparison of basal and resveratrol (RESV)-stimulated (100 μM) FdL activity of full-length human Sirt1-wt and Sirt1-T530D. (F) FdL deacetylation activity of full-length Sirt1 in the presence (100 μM) and the absence of the cyclic nucleotides cAMP and cGMP. The same set of experiments was done in the absence (left) and in the presence (right) of 100 μM resveratrol. The activity in the absence of any ligands was used as reference and set to 100%. (G) Effects of SRT1720 (1 μM) on the Sirt1-dependent deacetylation of FdL-peptide and of a p53-derived non-modified peptide.
