Introduction
The adoptive transfer of re-directed T cells that are engineered by gene transfer to express receptors that target tumor associated molecules and signal T cell effector functions is emerging as an effective modality for cancer therapy, and holds promise for treating a broad range of malignancies, including those that are commonly managed with autologous or allogeneic hematopoietic stem cell transplantation. This review will focus on current issues for engineering T cells with chimeric antigen receptors (CARs) that are specific for molecules on hematologic malignancies, and discuss the prospects and challenges for using such T cells either alone or as an adjunct to hematopoietic stem cell transplantation to reduce the unacceptably high rates of relapse associated with this procedure 1.
Chimeric Antigen Receptors – Target Molecules and Receptor Design
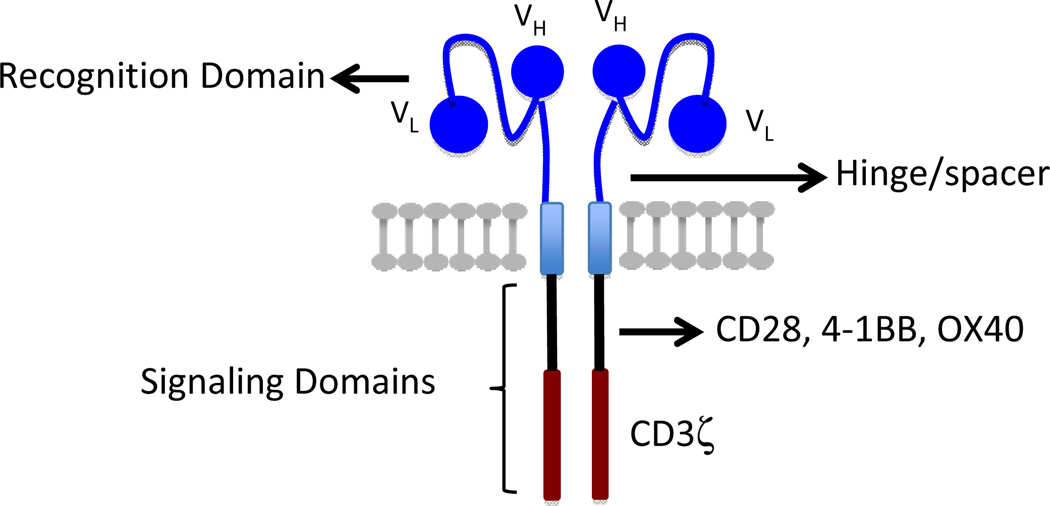
Genetic modification of T cells to confer tumor specificity surmounts many of the technical difficulties in isolating and expanding rare tumor-reactive T cells from patients, and can circumvent mechanisms that interfere with the antitumor activity of endogenous T cells by providing control over the specificity, avidity, function, and cellular composition of the antitumor immune response. A notable advance in the field of T cell engineering was the development of chimeric antigen receptors that link recognition domains of antibodies to molecules involved in signaling T cell effector function 2. CARs usually consist of a single chain variable fragment (scFV) derived from the VH and VL sequences of a monoclonal antibody specific for a tumor cell surface molecule, fused to sequences that encode a transmembrane domain, the CD3ζ signaling domain and one or more costimulatory signaling modules, such as CD28, 4-1BB, OX40, or CD27 (Figure 1) 3,4. Costimulation can also be provided in trans by encoding separately a ligand for a costimulatory receptor in the vector 5. Although an scFV is the most common tumor recognition domain for CARs, other molecules such as cytokines or peptides that bind to tumor cells have been incorporated as tumor targeting domains in the CAR design 6.
Figure 1.
Basic elements of chimeric antigen receptor design
CARs have been developed that encode scFvs specific for molecules expressed on several malignancies routinely treated with hematopoietic stem cell transplantation. These include the CD19, CD20 and ROR1 molecules expressed on B cell lymphomas and leukemias; CD33 and Lewis Y expressed on acute myeloid leukemia; and NKG2D ligands and Lewis Y expressed on multiple myeloma 7–12. One of the potential problems with the current targets is they do not completely distinguish normal from malignant cells, and on-target toxicity to normal cells expressing the molecule is an expected complication of therapy. This may be tolerated temporarily for lineage restricted molecules such as CD19 and CD20 that are also expressed on normal mature B cells and where intravenous immunoglobulin can be used to replace deficient antibody function. However, on target toxicity to normal tissues remains an issue for which novel strategies to mitigate toxicity such as by regulating CAR expression or T cell survival are needed. Exome sequencing, analysis of gene expression profiles, and elucidation of glycosylation of cell surface molecules on tumors versus normal cells may identify novel surface molecules or isoforms that can be selectively targeted on the malignancy without harming normal cells.
CARs, unlike conventional T cell receptors, are not MHC restricted and have the advantage that a single construct can be used to treat all patients that express the target molecule. However, CARs are synthetic and much remains to be understood concerning how to optimally design the receptor construct to ensure that T cells respond appropriately in vivo to tumor cells. The earliest CARs, termed “first generation”, linked the scFV to the CD3ζ chain and did not incorporate costimulation into the signaling module 13. These CARs entered clinical trials but significant antitumor activity was not observed 14,15. The lack of in vivo activity was attributed to absent costimulation in the CAR, although it must be acknowledged that methods for activating T cells, introducing the receptor constructs, and expanding cells for therapy have improved markedly. Recent studies have used efficient retroviral or lentiviral transduction to introduce CARs that contain one (second generation) or two (third generation) costimulatory domains into T cells and employed culture methods that rapidly expand T cells for adoptive transfer 16. T cells that express second and third generation CARs, or provide trans costimulation have more potent antitumor activity in vitro and in animal models 5,13,17. It must be conceded that the optimal design for clinical applications is still uncertain and may differ depending on the target molecule and malignancy being treated. These issues may eventually only be resolved by clinical trials that compare distinct constructs, both for efficacy and toxicity.
There are aspects of CAR design in addition to costimulation that may need to be considered including the density of target molecules on tumor cells required for effective T cell signaling, their proximity and accessibility on the tumor cell membrane, and the affinity of the scFv. In CLL for example, the number of cell surface CD19 molecules is several fold greater than that of ROR1, and how CAR occupancy might influence T cell signaling has not been carefully studied. The affinity of the scFv and steric constraints imposed by the location of the target epitope may require alterations in the extracellular spacer length to promote synapse formation between effector T cell and the tumor cell 18. Structural modeling of CARs interacting with their target molecules and measurement of on and off-rates of the CAR and tumor cell ligand may provide further insights into how to optimally design CARs specific for individual targets.
Clinical Applications of CAR-Modified T Cells
CAR engineered T cells have been tested for antitumor activity in pilot clinical trials in which autologous T cells have been removed from the patient, modified to express a CAR, and re-infused into the patient after expansion in vitro. Results of several trials of CARs specific for CD19, CD20, GD2, and other targets have been reported 19–24. Extremely promising results have been obtained targeting the B-cell lineage restricted CD19 molecule that is expressed on B-cell leukemias and lymphomas with CD19-specific CAR T cells 19–22,25. Kalos, Porter and June et al. reported durable remissions in patients with B cell chronic lymphocytic leukemia (CLL) after the infusion of autologous T cells transduced to express a CD19-specific CAR that contained a 4-1BB costimulatory domain. In these studies, the infusion of low doses of T cells led to in vivo expansion and persistence of CAR T cells, subsequent tumor lysis, and a sustained deficiency of normal CD19+ B cells 19. Significant antitumor activity, depletion of normal B cells, and side effects related to tumor lysis and cytokine release have also been reported in patients with CLL and lymphoma by groups at the National Institutes of Health, Memorial Sloan Kettering Cancer Center and Baylor College of Medicine in trials in which autologous T cells were modified to express CD19 CARs that contain a CD28 costimulatory domain 20,22,26. That durable responses can be achieved in patients with advanced B cell malignancies illustrates the potency of CAR-T cells, and suggests that future work to define optimal CD19 CAR constructs, and to integrate T cell therapy earlier after diagnosis or after autologous transplant where marked tumor cytoreduction can be achieved by intensive conditioning may improve outcome and lessen the toxicity resulting from CAR mediated tumor lysis.
The success of targeting CD19 on tumor cells predictably leads to the elimination of normal mature and immature B cells that also express CD19. Moreover, because CD19 is expressed when hematopoietic stem cells commit to the B cell lineage, no new B cells would be expected to emerge and depletion of B cells is likely to persist as long as the transferred T cells survive, express the CAR, and remain functional. Strategies to regulate survival of CAR T cells or turn off CAR expression are being developed as discussed below, and could allow the regeneration of normal B cells from hematopoietic progenitors. CD20 and ROR1 are alternative targets on B cell malignancies, however CD20 is also expressed on normal B cells and would be expected to result in B cell depletion. ROR1, which is uniformly expressed on CLL, mantle cell lymphoma and a subset of ALL, is not expressed on mature B cells in the periphery and could spare this compartment 8. ROR1 is transiently expressed on early B cell precursors in the bone marrow and on some normal tissues, thus toxicity remains a concern with targeting ROR1. Animal studies are in progress to determine if ROR1 is a safe target for CAR therapy.
CAR-modified T cells may also have applications in solid tumors, including those in children that can be managed with high dose therapy and autologous stem cell transplantation. Candidate surface molecules on solid tumors, including mesothelin, folate receptor, L1CAM, and GD2 have been identified and are being developed for clinical applications. Significant antitumor activity without toxicity has been reported in patients with neuroblastoma treated with T cells modified with a first generation GD2 CAR 27. The persistence of the transferred cells was relatively short in this study, perhaps related to the lack of costimulation in the CAR. GD2 is expressed on normal peripheral nerves and on-target toxicity from a sustained T cell response will need to be monitored if more potent CARs are employed 24.
Composition of CAR-T Cell Products
The initial clinical applications of CAR-T cells have primarily derived cell products for administration by inserting the CAR gene into unselected polyclonal T cells obtained from the patient by leukapheresis. It is now well established that peripheral T cell pool contains different proportions of naïve and memory CD4+ and CD8+ cell subsets, and that these subsets diverge in function, transcriptional profiles and epigenetic programming, with potential consequences for their efficacy in adoptive therapy 28. Direct comparisons have revealed profound differences in the ability of T cells from different subsets to persist long term in vivo and revert to the memory pool. For example, in animal models the adoptive transfer of CD8+ CD62L+ central memory T cells (TCM) or a rare subset of CD62L+ memory T cells (termed memory stem cells; TSCM) that has cell surface markers shared by both naïve (TN) and TCM cells, exhibit superior survival in vivo and/or mediate superior antitumor activity compared with more differentiated CD62L− effector memory (TEM) cells 29–31. CD8+ TN cells are undifferentiated, have long telomeres and substantial proliferative capacity, and as a consequence might be directed by cytokines or small molecules during in vitro expansion and transduction to have properties that confer superior antitumor activity 32. The CD4+ T cell compartment is also heterogeneous, with defined naïve, memory and FoxP3+ regulatory subsets 33. CD4+ T cells alone have been shown to exhibit antitumor activity after adoptive transfer in animal models and humans, at least in part by providing help for CD8+ T cells 34, and the potential to formulate CAR-modified T cell products that contain defined proportions of CD8+ and CD4+ T cells from distinct subsets is beginning to be explored. Because the phenotypic distribution of T cells in the blood is influenced by age, pathogen exposure and prior chemotherapy, the use of unselected populations for gene transduction results in a lack of uniformity in the composition of T cell products administered to individual patients, and could affect potency and toxicity. The disadvantage of having to select distinct subsets for therapy is the additional cost and time associated with deriving the cell product for therapy. Transduction of unselected T cells should provide some proportion of T cells with the desired traits, and for malignancies that are sensitive to immunotherapy, may be sufficient for efficacy. However, differences in the composition of cell products will make it difficult to define dose/response relationships and to ensure immediate potency against less responsive tumors.
Incorporating CAR-T Cells Into Stem Cell Transplant Regimens
The antitumor activity of autologous CD19 CAR-T cells in patients with advanced B cell malignancies, illustrates the potential to incorporate the administration of T cells modified with a CAR to reduce relapse after autologous stem cell transplant, and to utilize donor T cells modified with a CAR to augment the graft versus leukemia (GVL) effect of allogeneic HCT. The importance of lymphodepleting chemoradiotherapy for improving the persistence and antitumor efficacy of transferred T cells was established in studies in melanoma 35, and suggests that intensive conditioning administered before autologous HCT could provide an ideal environment for administering CAR-T cells. Studies targeting CD19 in lymphoma patients undergoing autologous HCT are in progress and the results will be of interest. If effective, this could renew efforts to use intensive conditioning and autologous HCT combined with T cell immunotherapy for selected solid tumors that express unique cell surface molecules for which CARs have been designed.
Leukemia relapse remains a major cause of failure after allogeneic HCT and the long sought goal of augmenting the graft versus leukemia (GVL) effect without aggravating graft versus host disease remains elusive. This could potentially be accomplished using CAR-T cells, however in this setting the composition of CAR-T cell products will be critical to avoid infusing alloreactive T cells that can cause GVHD. One strategy involves selecting donor CD8+ cytomegalovirus (CMV) or Epstein Barr virus (EBV)-specific T cells that have been administered without GVHD in prior studies 36, and engineering these cells to express the tumor-reactive CAR 37. In principle, virus-specific CD4+ T cells could be similarly modified if it essential for therapeutic efficacy to have both subsets in the cell product. Clinical trials of CD19 CAR-modified T cells following allogeneic HCT are now accruing patients, and should assist in defining both the potential for efficacy and limitations of this approach in patients with B cell malignancies. Ideally, CARs specific for molecules on myeloid leukemias will soon be validated and advance to clinical testing to provide therapeutic options for this subset of patients.
Modification to enhance safety and efficacy of CAR-T cells
Because most of the current CARs do not completely discriminate normal and malignant cells, strategies to improve safety are a significant area of research. A conditional suicide gene that encodes human caspase 9 fused to a modified human FK-binding protein confers sensitivity to a synthetic small molecule that induces apoptosis through activation of caspase 9. This approach effectively ablated alloreactive T cells in a clinical trial after human hematopoietic cell transplant, and may prove useful to abrogate on-target or off-target toxicities of CAR engineered T cells 38. Expressing CARs through RNA transfection of T cells ensures a gradual loss of surface CAR expression as T cells divide and may be a useful strategy to define potential toxicity of novel constructs 39. Another approach is to regulate expression of the tumor-targeting CAR by placing it under the control of regulatory elements that can be turned on (or off) by delivering a small molecule 40.
Strategies to improve efficacy are also likely to be necessary as clinical trials are extended to more aggressive tumors. Genetic strategies to overcome local tumor immunosuppression mediated by stromal cells, cytokines, and negative signaling pathways in T cells have been devised. One approach expresses a single chain IL-12 molecule in tumor-specific T cells to re-program tumor associated myeloid cells and eliminate the need for lymphodepleting chemotherapy prior to CAR-T cell therapy 41. Gene editing technology could allow permanent disruption of negative signaling pathways such as PD-1, although combinatorial approaches utilizing blocking antibodies that themselves have some efficacy may be a more convenient approach 42. The development of strategies to enhance efficacy will best be accomplished by careful analysis of treated patients to define the basis for success and failure.
Conclusions
Immunotherapy for cancer has undergone a renaissance in recent years, based both on technologic developments such as the ease in introducing genes into T cells and on an improved understanding of the obstacles to eradicating cancer through immune mechanisms. Although much remains to be discovered, there is considerable optimism that this modality can have a meaningful impact for patients, both alone and as an adjunct to hematopoietic stem cell transplant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bacher U, Talano JA, Bishop MR. Monitoring and prevention of relapse after allogeneic hematopoietic cell transplantation for myeloid malignancies. Biology of Blood and Marrow Transplantation. 2012;18(1 Suppl):S62–S73. doi: 10.1016/j.bbmt.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 2.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T cell receptors. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(2):720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3(1):35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 4.Song DG, Ye Q, Poussin M, Harms GM, Figini M, Powell DJ., Jr CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood. 2012;119(3):696–706. doi: 10.1182/blood-2011-03-344275. [DOI] [PubMed] [Google Scholar]
- 5.Stephan MT, Ponomarev V, Brentjens RJ, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nature Medicine. 2007;13(12):1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 6.Brown CE, Starr R, Aguilar B, et al. Stem-like Tumor-Initiating Cells Isolated from IL13Ralpha2 Expressing Gliomas Are Targeted and Killed by IL13-Zetakine-Redirected T Cells. Clinical Cancer Research. 2012;18(8):2199–2209. doi: 10.1158/1078-0432.CCR-11-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper LJ, Topp MS, Serrano LM, et al. T cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101(4):1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 8.Hudecek M, Schmitt TM, Baskar S, et al. The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood. 2010;116(22):4532–4541. doi: 10.1182/blood-2010-05-283309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen MC, Clarke P, Tan G, et al. Human T lymphocyte genetic modification with naked DNA. Molecular Therapy. 2000;1(1):49–55. doi: 10.1006/mthe.1999.0012. [DOI] [PubMed] [Google Scholar]
- 10.Peinert S, Prince HM, Guru PM, et al. Gene-modified T cells as immunotherapy for multiple myeloma and acute myeloid leukemia expressing the Lewis Y antigen. Gene Therapy. 2010;17(5):678–686. doi: 10.1038/gt.2010.21. [DOI] [PubMed] [Google Scholar]
- 11.Dutour A, Marin V, Pizzitola I, et al. In Vitro and In Vivo Antitumor Effect of Anti-CD33 Chimeric Receptor-Expressing EBV-CTL against CD33 Acute Myeloid Leukemia. Advances in Hematology. 2012;2012:683065. doi: 10.1155/2012/683065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber A, Meehan KR, Sentman CL. Treatment of multiple myeloma with adoptively transferred chimeric NKG2D receptor-expressing T cells. Gene Therapy. 2011;18(5):509–516. doi: 10.1038/gt.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Research. 2006;66(22):10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 14.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112(6):2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JR, Digiusto DL, Slovak M, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15(4):825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 16.June CH. Principles of adoptive T cell cancer therapy. Journal of Clinical Investigation. 2007;117(5):1204–1212. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kershaw MH, Teng MW, Smyth MJ, Darcy PK. Supernatural T cells: genetic modification of T cells for cancer therapy. Nature Reviews Immunology. 2005;5(12):928–940. doi: 10.1038/nri1729. [DOI] [PubMed] [Google Scholar]
- 18.James SE, Greenberg PD, Jensen MC, et al. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. Journal of Immunology. 2008;180(10):7028–7038. doi: 10.4049/jimmunol.180.10.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science Translational Medicine. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England Journal of Medicine. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118(18):4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Till BG, Jensen MC, Wang J, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119(17):3940–3950. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. The Journal of Clinical Investigation. 2011;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nature Medicine. 2008;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weng NP, Araki Y, Subedi K. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nature Reviews Immunology. 2012;12(4):306–315. doi: 10.1038/nri3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. The Journal of Clinical Investigation. 2008;118(1):294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, Jensen MC. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood. 2011;117(6):1888–1898. doi: 10.1182/blood-2010-10-310599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nature Medicine. 2011;17(10):1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nature Reviews Immunology. 2012;12(4):269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zygmunt B, Veldhoen M. T helper cell differentiation more than just cytokines. Advances in Immunology. 2011;109:159–196. doi: 10.1016/B978-0-12-387664-5.00005-4. [DOI] [PubMed] [Google Scholar]
- 34.Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. The New England Journal of Medicine. 2008;358(25):2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. Journal of Clinical Oncology. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terakura S, Yamamoto TN, Gardner RA, Turtle CJ, Jensen MC, Riddell SR. Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood. 2012;119(1):72–82. doi: 10.1182/blood-2011-07-366419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Stasi A, Tey SK, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. The New England Journal of Medicine. 2011;365(18):1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrett DM, Zhao Y, Liu X, et al. Treatment of advanced leukemia in mice with mRNA engineered T cells. Human Gene Therapy. 2011;22(12):1575–1586. doi: 10.1089/hum.2011.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buchholz DR. Tet-on binary systems for tissue-specific and inducible transgene expression. Methods in Molecular Biology. 2012;917:265–275. doi: 10.1007/978-1-61779-992-1_16. [DOI] [PubMed] [Google Scholar]
- 41.Pegram HJ, Lee JC, Hayman EG, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119(18):4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England Journal of Medicine. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]