Fig. 7.
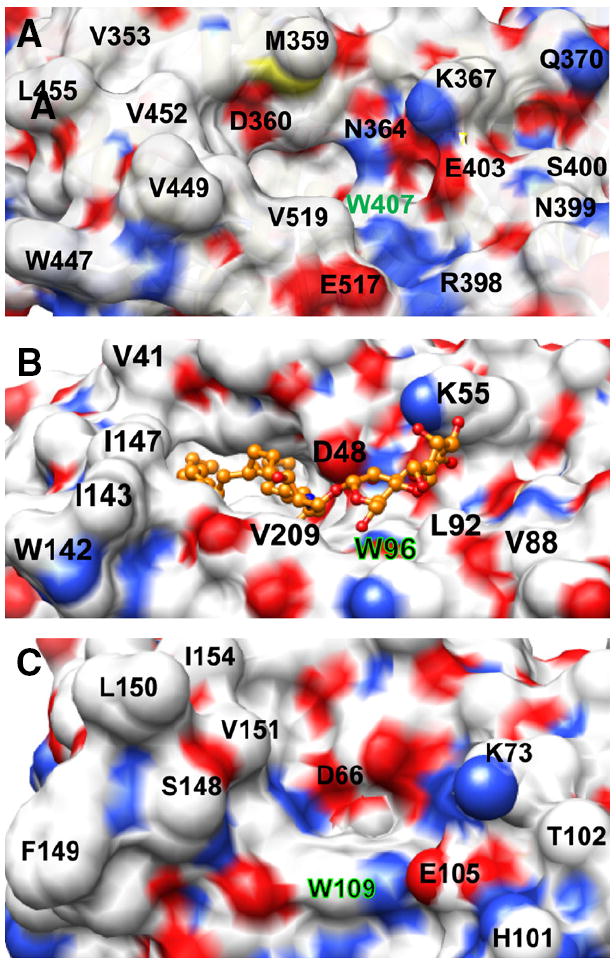
Surface topology of the sugar headgroup recognition site of FAPP2-C212, GLTP, and HET-C2. Surface residue color reflects the charge status (red = negative, blue = positive, and white = neutral). The Trp residue that interacts directly with the initial ceramide-linked GSL sugar in each GLTP-fold is accentuated by green highlights. A) FAPP2-C212. Residues such as Glu403, Val519, Val397, Arg398, Asn399, and Ser400 partially obstruct the surface near Trp407 and constrict the sugar headgroup binding site. B) Human GLTP (PDB ID: 1SX6). 18:1-LacCer is depicted in gold with the ceramide hydrocarbon chains (left side) disappearing into the hydrophobic pocket. The open, unobstructed surface adjacent and to the right of Trp96 allows for broader selectivity for binding of various glycolipids. C) Fungal HET-C2 (PDB ID: 3KV0). Residues such as Glu105, Thr102, His101, and Trp208 adjacent to Trp109 act as obstructions and create a pit-like sugar headgroup binding site.
