Abstract
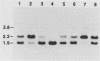
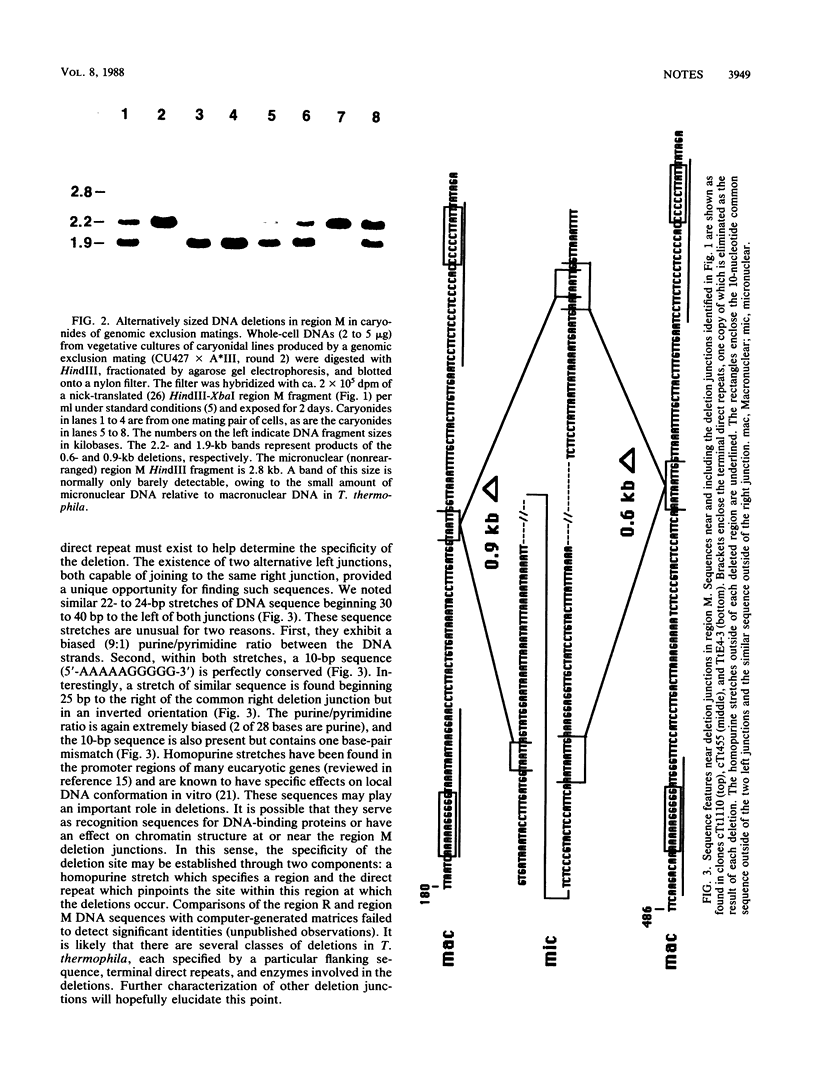
Deletions of specific DNA sequences are known to occur in Tetrahymena thermophila as a developmentally regulated process. Deletions of a particular region (region M) were previously shown to be of two alternative sizes, 0.6 or 0.9 kilobases (kb) (C.F. Austerberry, C.D. Allis, and M.-C. Yao, Proc. Natl. Acad. Sci. USA 81: 7383-7387). In this study, the nucleotide sequences for both deletions were determined. These two deletions share the same right junction, but their left junctions are 0.3 kb apart. An 8-base-pair (bp) sequence is present at both junctions of the 0.6-kb deletion, but only 5 bp of this direct repeat are present at the left junction of the 0.9-kb deletion. Further comparison revealed a common 10-bp sequence near each of the two left junctions and a similar sequence in inverted orientation near the right junction. These sequences may play a role in the developmental regulation of the deletion process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. L. Cytogenetics of genomic exclusion in Tetrahymena. Genetics. 1967 Apr;55(4):797–822. doi: 10.1093/genetics/55.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Blackwell T. K., DePinho R. A., Reth M. G., Yancopoulos G. D. Regulation of genome rearrangement events during lymphocyte differentiation. Immunol Rev. 1986 Feb;89:5–30. doi: 10.1111/j.1600-065x.1986.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Austerberry C. F., Allis C. D., Yao M. C. Specific DNA rearrangements in synchronously developing nuclei of Tetrahymena. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7383–7387. doi: 10.1073/pnas.81.23.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austerberry C. F., Yao M. C. Nucleotide sequence structure and consistency of a developmentally regulated DNA deletion in Tetrahymena thermophila. Mol Cell Biol. 1987 Jan;7(1):435–443. doi: 10.1128/mcb.7.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon G. A., Bowen J. K., Yao M. C., Gorovsky M. A. Tetrahymena H4 genes: structure, evolution and organization in macro- and micronuclei. Nucleic Acids Res. 1984 Feb 24;12(4):1961–1975. doi: 10.1093/nar/12.4.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H., Karrer K. M. Genomic reorganization in ciliated protozoans. Annu Rev Genet. 1986;20:501–521. doi: 10.1146/annurev.ge.20.120186.002441. [DOI] [PubMed] [Google Scholar]
- Borst P., Greaves D. R. Programmed gene rearrangements altering gene expression. Science. 1987 Feb 6;235(4789):658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- Callahan R. C., Shalke G., Gorovsky M. A. Developmental rearrangements associated with a single type of expressed alpha-tubulin gene in Tetrahymena. Cell. 1984 Feb;36(2):441–445. doi: 10.1016/0092-8674(84)90237-x. [DOI] [PubMed] [Google Scholar]
- Cupples C. G., Pearlman R. E. Isolation and characterization of the actin gene from Tetrahymena thermophila. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5160–5164. doi: 10.1073/pnas.83.14.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J. C., Cartwright I. L., Thomas G. H., Elgin S. C. Selected topics in chromatin structure. Annu Rev Genet. 1985;19:485–536. doi: 10.1146/annurev.ge.19.120185.002413. [DOI] [PubMed] [Google Scholar]
- Horowitz S., Bowen J. K., Bannon G. A., Gorovsky M. A. Unusual features of transcribed and translated regions of the histone H4 gene family of Tetrahymena thermophila. Nucleic Acids Res. 1987 Jan 12;15(1):141–160. doi: 10.1093/nar/15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz S., Gorovsky M. A. An unusual genetic code in nuclear genes of Tetrahymena. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2452–2455. doi: 10.1073/pnas.82.8.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard E. A., Blackburn E. H. Reproducible and variable genomic rearrangements occur in the developing somatic nucleus of the ciliate Tetrahymena thermophila. Mol Cell Biol. 1985 Aug;5(8):2039–2050. doi: 10.1128/mcb.5.8.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobutcher L. A., Jahn C. L., Prescott D. M. Internal sequences are eliminated from genes during macronuclear development in the ciliated protozoan Oxytricha nova. Cell. 1984 Apr;36(4):1045–1055. doi: 10.1016/0092-8674(84)90054-0. [DOI] [PubMed] [Google Scholar]
- Kohwi-Shigematsu T., Kohwi Y. Poly(dG)-poly(dC) sequences, under torsional stress, induce an altered DNA conformation upon neighboring DNA sequences. Cell. 1985 Nov;43(1):199–206. doi: 10.1016/0092-8674(85)90024-8. [DOI] [PubMed] [Google Scholar]
- Kraut H., Lipps H. J., Prescott D. M. The genome of hypotrichous ciliates. Int Rev Cytol. 1986;99:1–28. doi: 10.1016/s0074-7696(08)61422-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nielsen H., Andreasen P. H., Dreisig H., Kristiansen K., Engberg J. An intron in a ribosomal protein gene from Tetrahymena. EMBO J. 1986 Oct;5(10):2711–2717. doi: 10.1002/j.1460-2075.1986.tb04555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Aparicio R. M., Sparkowski J. J., Proulx A. E., Mitchell J. D., Klobutcher L. A. Nucleic acid splicing events occur frequently during macronuclear development in the protozoan Oxytricha nova and involve the elimination of unique DNA. Genes Dev. 1987 Jun;1(4):323–336. doi: 10.1101/gad.1.4.323. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinbrück G. Molecular reorganization during nuclear differentiation in ciliates. Results Probl Cell Differ. 1986;13:105–174. doi: 10.1007/978-3-540-39838-7_3. [DOI] [PubMed] [Google Scholar]
- White T. C., Allen S. L. Alternative processing of sequences during macronuclear development in Tetrahymena thermophila. J Protozool. 1986 Feb;33(1):30–38. doi: 10.1111/j.1550-7408.1986.tb05551.x. [DOI] [PubMed] [Google Scholar]
- Wu M., Allis C. D., Richman R., Cook R. G., Gorovsky M. A. An intervening sequence in an unusual histone H1 gene of Tetrahymena thermophila. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8674–8678. doi: 10.1073/pnas.83.22.8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Choi J., Yokoyama S., Austerberry C. F., Yao C. H. DNA elimination in Tetrahymena: a developmental process involving extensive breakage and rejoining of DNA at defined sites. Cell. 1984 Feb;36(2):433–440. doi: 10.1016/0092-8674(84)90236-8. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Gorovsky M. A. Comparison of the sequences of macro- and micronuclear DNA of Tetrahymena pyriformis. Chromosoma. 1974;48(1):1–18. doi: 10.1007/BF00284863. [DOI] [PubMed] [Google Scholar]
- Yokoyama R., Yao M. C. Internal micronuclear DNA regions which include sequences homologous to macronuclear telomeres are deleted during development in Tetrahymena. Nucleic Acids Res. 1984 Aug 10;12(15):6103–6116. doi: 10.1093/nar/12.15.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]