Table 2. Structure–Activity Investigations of the 2-Fluoro Alkyne Agonists.
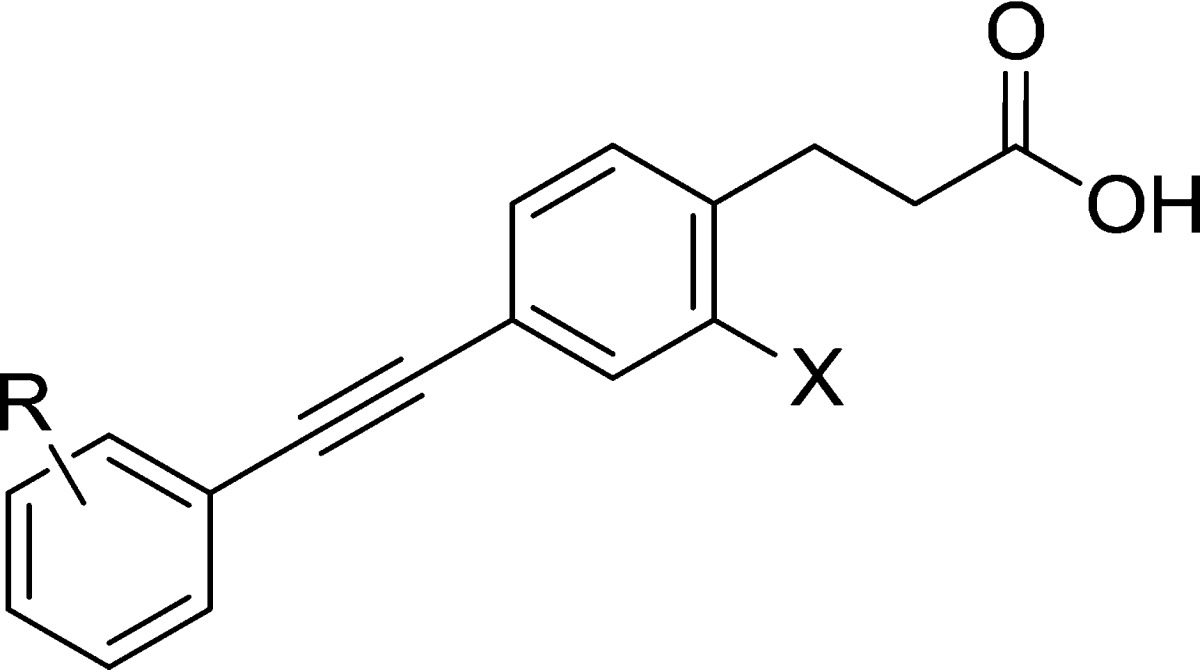
| pEC50 (efficacy,
%) |
|||||||
|---|---|---|---|---|---|---|---|
| compd | R1 | X | hFFA1, calciuma | hFFA4, BRETb | ClogPc | LEd | LLEe |
| 9 | 2-Me | H | 7.34 ± 0.07 (103) | 5.84 ± 0.01 (103) | 5.04 | 0.50 | 2.30 |
| 10 | 2-Me | F | 7.48 ± 0.03 (107) | 5.80 ± 0.03 (98) | 5.18 | 0.49 | 2.30 |
| 11 | 3-Me | F | 7.65 ± 0.03 (100) | 5.41 ± 0.07 (124) | 5.18 | 0.50 | 2.47 |
| 12 | 2-Me, 5-CN | F | 7.77 ± 0.03 (104) | 5.02 ± 0.04 (123) | 4.62 | 0.46 | 3.15 |
| 13 | 2-CF2H, 5-F | F | 7.74 ± 0.04 (97) | 5.93 ± 0.04 (117) | 5.02 | 0.44 | 2.72 |
| 14 | 3,5-Cl | F | 7.42 ± 0.07 (99) | 5.50 ± 0.25 (90) | 6.11 | 0.46 | 1.31 |
| 15 | 2-CH2Ms | F | 5.84 ± 0.02 (97) | n.a. | 2.67 | 0.33 | 3.17 |
| 16 | 3-CH2Ms | F | 5.71 ± 0.02 (104) | 4.19 ± 0.06 (26) | 2.67 | 0.33 | 3.04 |
| 17 | 2-CH2O(CH2)2Ms | F | 6.21 ± 0.03 (94) | n.a. | 3.33 | 0.31 | 2.88 |
| 18 | 3-CH2O(CH2)2Ms | F | 6.44 ± 0.03 (93) | n.a. | 3.33 | 0.33 | 3.11 |
| 19 | 2-CH2O(CH2)3Ms | F | 6.14 ± 0.04 (92) | n.a. | 3.59 | 0.30 | 2.55 |
| 20 | 3-CH2O(CH2)3Ms | F | 6.43 ± 0.04 (83) | n.a. | 3.59 | 0.31 | 2.84 |
| 21 | 2-CH2CN | H | 7.70 ± 0.04 (103) | 6.11 ± 0.06 (99) | 3.96 | 0.48 | 3.76 |
| 22 | 2-CH2CN | F | 8.21 ± 0.03 (102) | 6.03 ± 0.06 (98) | 4.11 | 0.49 | 4.10 |
| 23 | 3-CH2CN | F | 7.13 ± 0.03 (104) | 5.41 ± 0.07 (115) | 4.11 | 0.42 | 3.02 |
| 24 | 2-CH2CH2CN | F | 7.74 ± 0.04 (97) | 5.86 ± 0.00 (114) | 4.25 | 0.44 | 3.50 |
Efficacy is given as % response relative to 10 μM TUG-20.19
Efficacy is given as % response relative to 9; n.a. = no activity (pEC50 < 4).14
Calculated by BioByte’s algorithm as implemented in ChemBioDraw Ultra 12.0 (ClogP option).
LE = RTln KD, presuming that EC50 ≈ KD. Values are given in kcal mol–1 per non-hydrogen atom.17
LLE = pEC50 – ClogP.18
