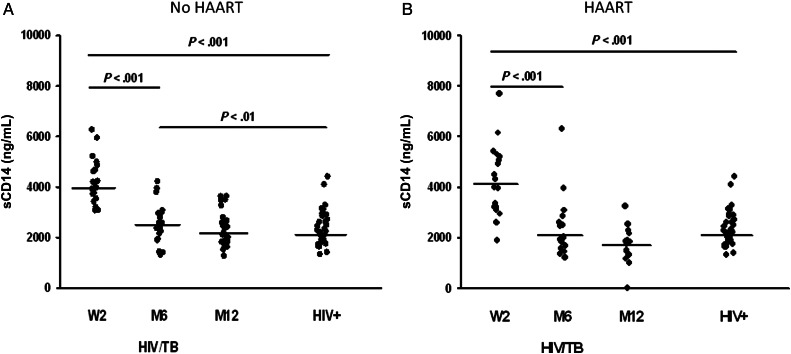
Figure 4.
Circulating soluble (s) CD14 in dual HIV/tuberculosis-infected subjects with high CD4 T-cell count (≥350/μL). Plasma from HIV/tuberculosis subjects who were treated with 6 months of anti-tuberculosis treatment alone (A) or who received HAART in addition to anti-tuberculosis treatment (B) were assessed for sCD14 at time of HAART randomization (W2), at 6 months (M6), and at 12 months (M12). Plasma sCD14 in a group of healthy CD4-matched HIV-positive subjects (HIV+) is shown for comparison. Line shown in each group represents median sCD14 activity. Abbreviations: HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus.