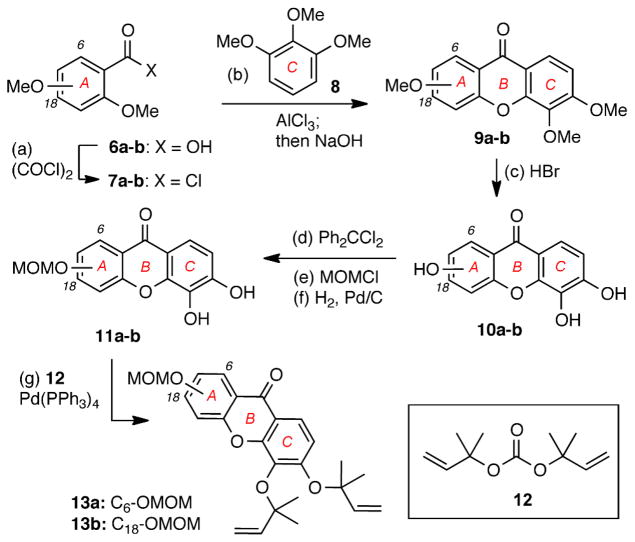
Scheme 1.
Reagents and conditions: (a) 5.0 equiv. oxalyl chloride, DMF (cat.), DCM, 16 h, 25 °C; (b) 3.0 equiv. AlCl3, Et2O, 18 h, 25 °C then 9.0 equiv NaOH, MeOH:H2O (1.5:1), 48 h, 110 °C, 90% for 9a, 58% for 9b; (c) 48% HBr: HOAc (1:2), 12 h, 120 °C, 95% for 10a, 81% for 10b; (d) 1.6 equiv. Ph2CCl2, Ph2O, 4 h, 175 °C; (e) 2.5 equiv. MOMCl, 2.5 equiv NaH, acetone, 12 h, 25 °C; (f) H2, 10% Pd/C, THF:MeOH (3:1), 18 h, 25 °C, 56% for 11a; 43% for 11b (over 3 steps); (g) 10 equiv. 12, 10 mol% Pd(PPh3)4, THF, 2 h, 5 °C, 63% for 13a, 84% for 13b.