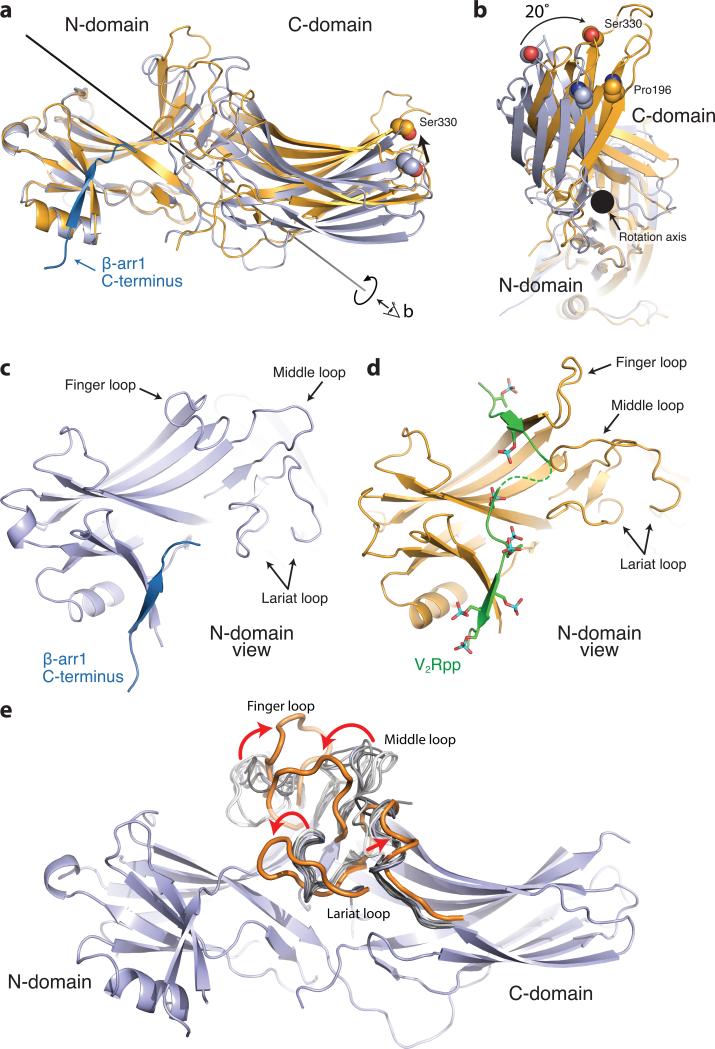
Figure 2. Conformational changes associated with β-arrestin1 activation.
The structures of inactive β-arrestin1 (PDB ID: 1G4M chain A, light blue) and active β-arrestin1 (gold) were aligned on the N-domains. The β-arrestin1 carboxy terminus is highlighted in dark blue. a, A substantial rotation and translation of the C-domain relative to the N-domain occurs upon activation. The rotation axis is indicated as a solid black line. b, View of C-domain rotation along the axis. c, N-domain of inactive arrestin, highlighting important regions. d, Active β-arrestin1 in the same orientation, showing V2Rpp in green. Phosphorylated residues are highlighted as sticks. e, The overall structure of inactive β-arrestin1 (PDB ID: 1G4M, chain A), with loops from all inactive β-arrestin1 structures superimposed (grey loops). The active conformation of these loops (orange loops) deviates from all inactive structures.