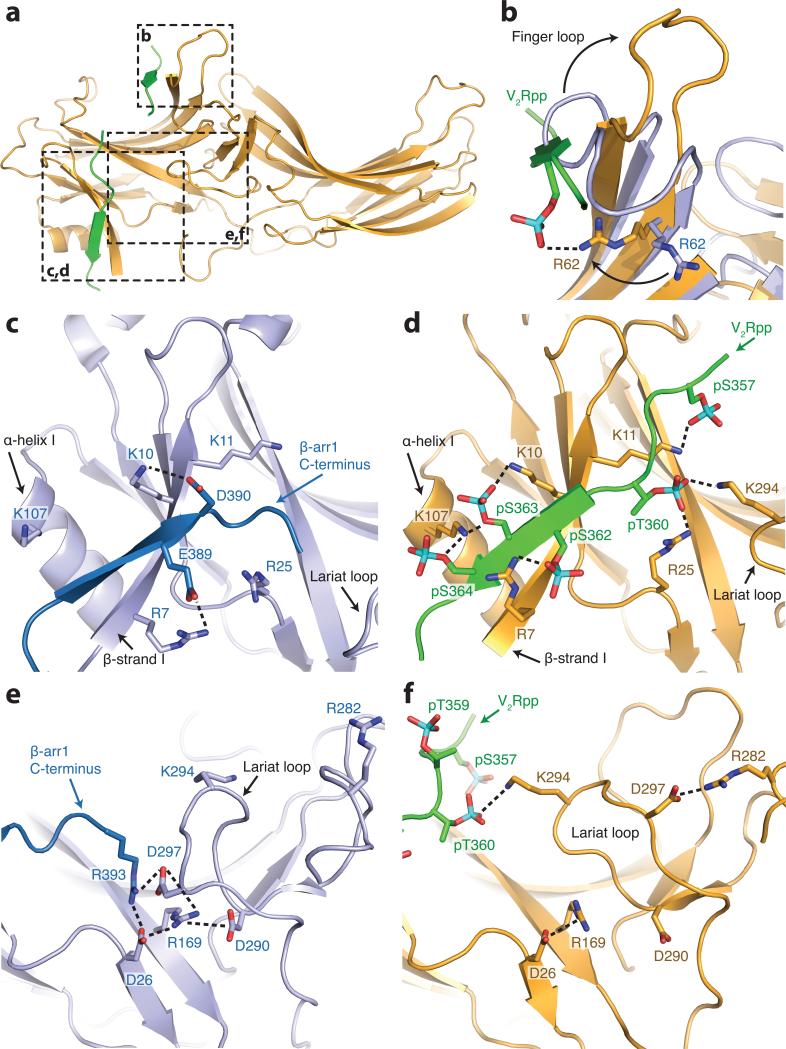
Figure 3. V2Rpp interactions with β-arrestin1.
a, Overall view of β-arrestin1, with regions of interest in boxes. Select charge-charge contacts are shown in dotted lines. b, V2Rpp (green) displaces the inactive finger loop (light blue), causing it to adopt an extended conformation in the active state (gold). c, In the inactive conformation, the β-arrestin1 carboxy-terminal β strand (dark blue) lies along the N-domain in the “three element” interaction network. d, Upon activation, this strand is displaced by the carboxy terminus of the V2Rpp, which engages in extensive charge-charge interactions through phosphorylated residues. e, The “polar core” of β-arrestin1 is thought to be a critical stabilizer of the inactive state. f, Upon V2Rpp binding, the carboxy-terminal strand residue Arg393 is displaced, and its interaction partner D297 undergoes a large movement together with the rest of the lariat loop.